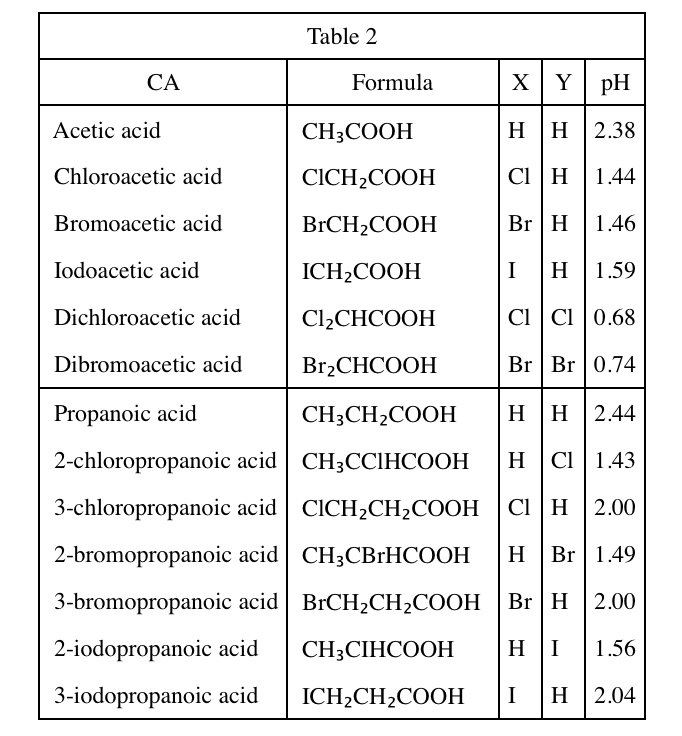
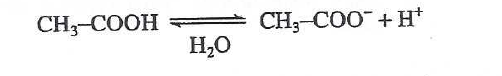39. According to Table 2, a 1.00 M aqueous solution of which CA, acetic acid or propanoic acid, is more acidic at 25℃?
Your Answer is
Correct Answer is B
Explanation
The lower the pH value, the stronger the acidity. According to table 2, the pH value of acetic acid is 2.38, which is less than 2.44 of propanoic acid, so the acidity of acetic acid is stronger