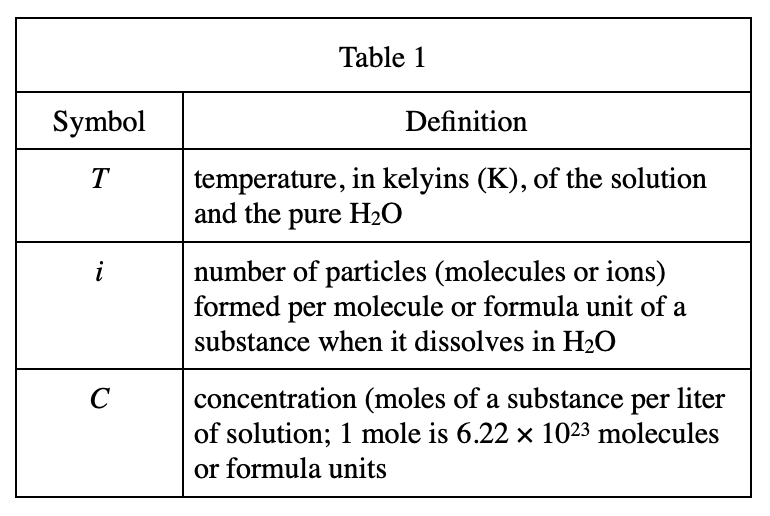
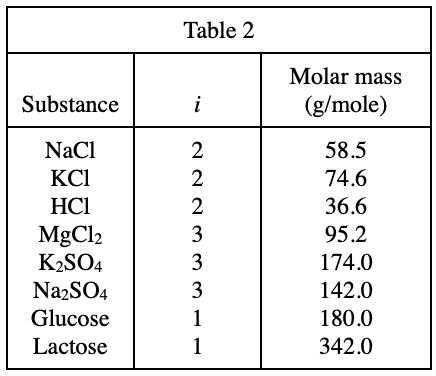
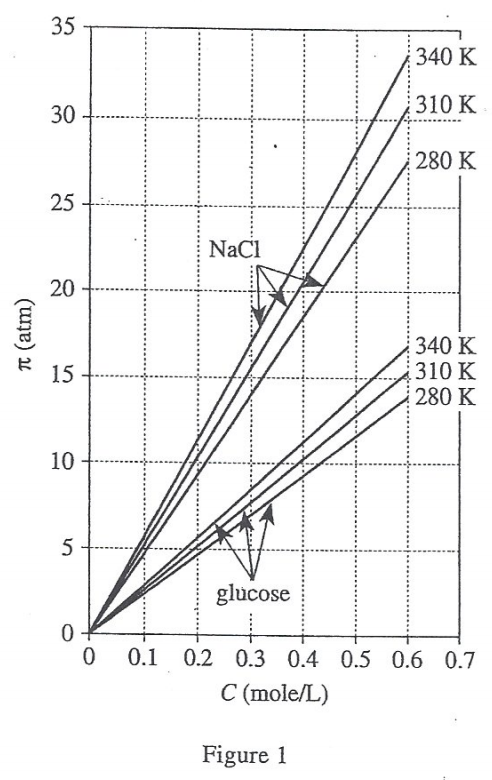
36. Consider a solution for which C = 0.20 mole/L and π = 10 atm. Based on Figure 1, this solution is most likely an aqueous solution of:
Your Answer is
Correct Answer is G
Explanation
Look at figure 1, when the abscissa C=0.20 and the ordinate π=10, the modified line segment should be NaCl at 310 K