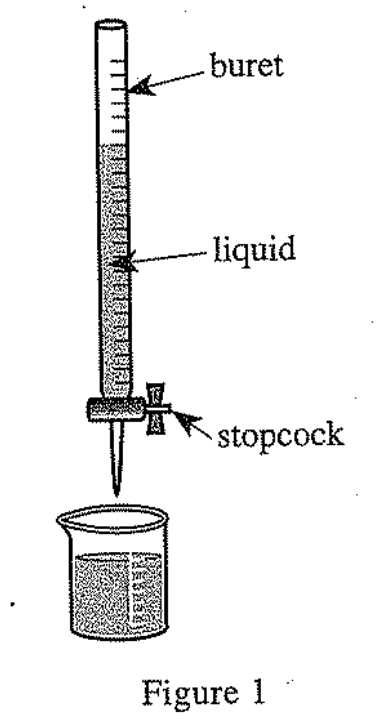
22. Which of the following graphs of the relative viscosities of DMSO, H2O, and isopropanol is most consistent with Student 3's explanation?
Your Answer is
Correct Answer is H
Explanation
Viscosity viscosity, according to common sense, the higher the viscosity, the slower the flow.
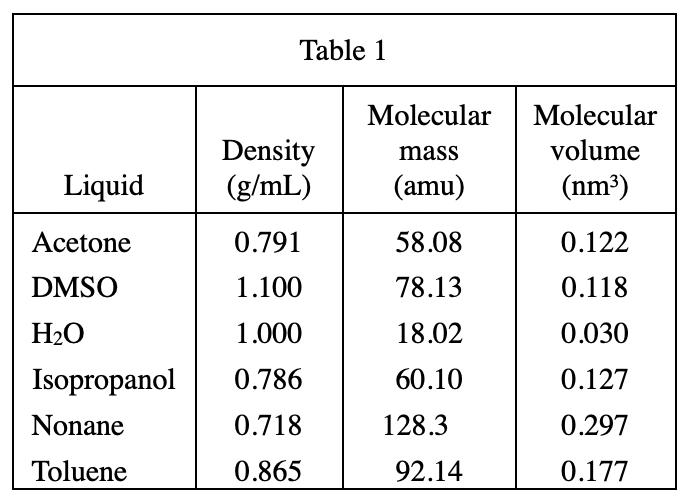
Look at the last three lines of student 3 Thus, if..., the liquid with the smaller molecular volume will always flow more easily. That is to say, the smaller the molecular volume, the faster the flow rate, that is, the smaller the viscosity.
Look at table 1 again, the molecular mass of DMSO=0.118, H2O=0.030, isopropanol=0.127, so the viscosity of H2O is the smallest , the viscosity of isopropanol is the largest