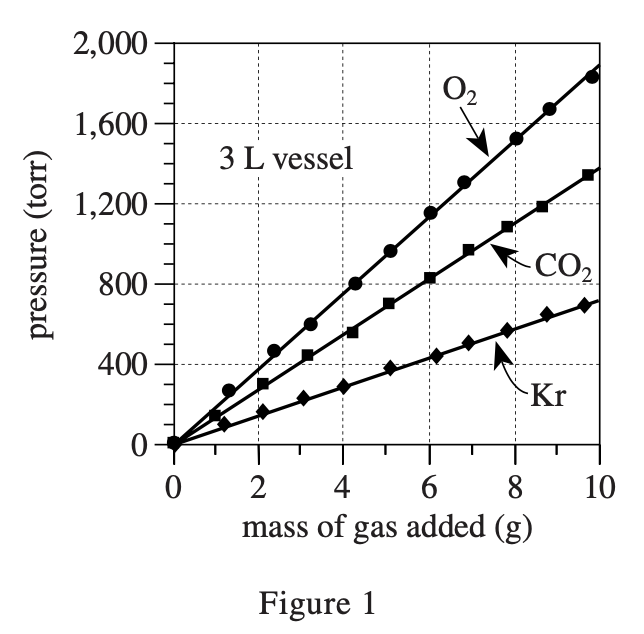
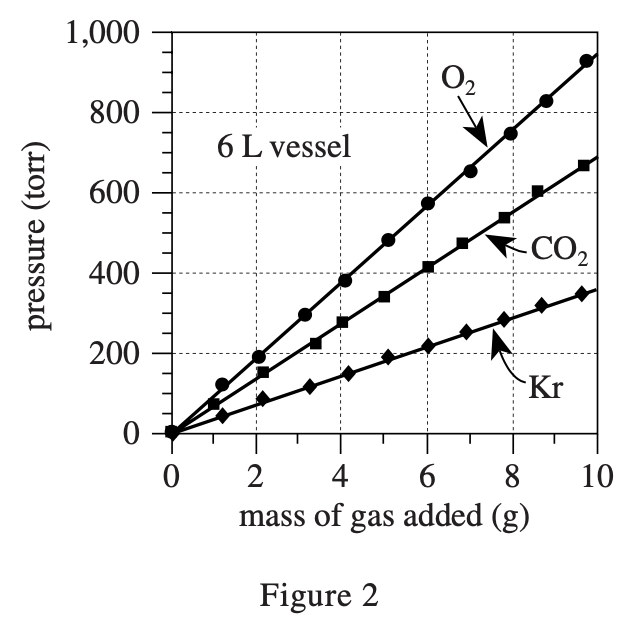
35. Suppose the experiment involving O2 and the 6 L vessel had been repeated, except at a room temperature of 14°C. For a given mass of O2, compared to the pressure measured in the original experiment, the pressure measured at 14°C would have been:
Your Answer is
Correct Answer is A
Explanation
The gas pressure formula in a closed container is P=nRT/V, where n is the number of molecules, R is a constant, T is the temperature, and V is the volume of the container.
Look at the formula, the lower the temperature, the smaller the pressure