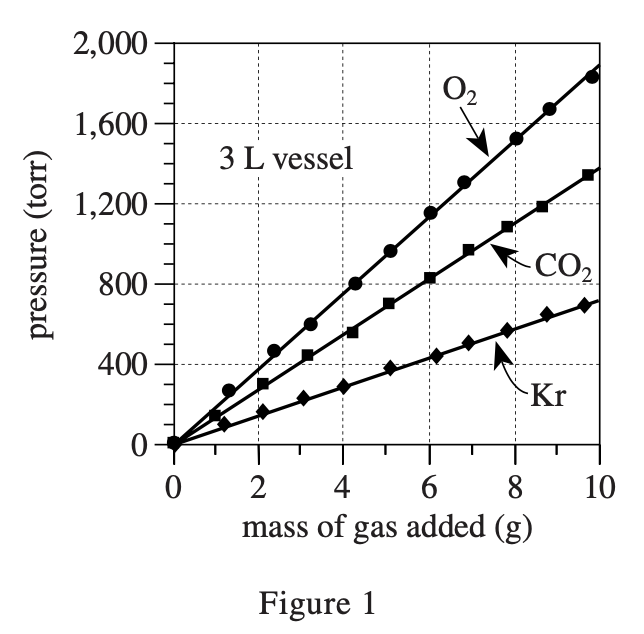
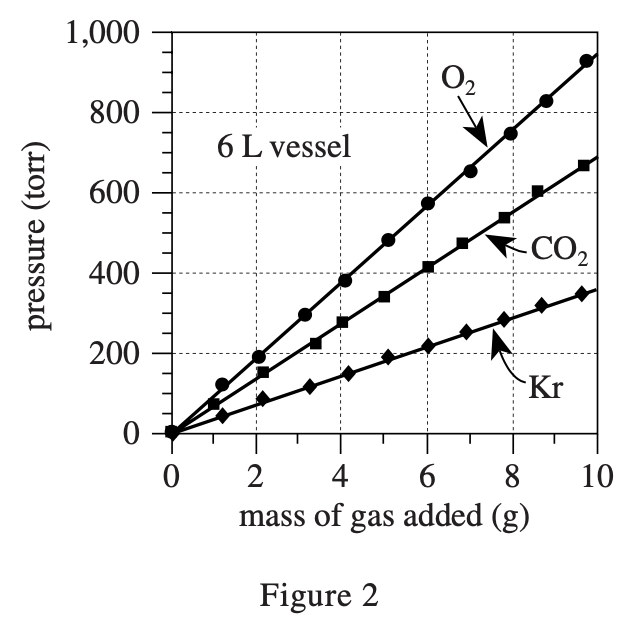
34. Which of the following best explains why equal masses of O2 and CO2 at the same temperature and in the same-size vessel had different pressures? The pressure exerted by the O2 was:
Your Answer is
Correct Answer is J
Explanation
The formula for gas pressure in a closed container is P=nRT/V, where n is the number of molecules, R is a constant, T is the temperature, and V is the volume of the container.
The greater the number of molecules, the greater the P. CO2 and O2 with the same mass, O2 has more molecules (because CO2 The molecular mass is 44, which is larger than the 32 of O2, so to achieve the same mass, you need more O2 molecules), O2 >The pressure is strong