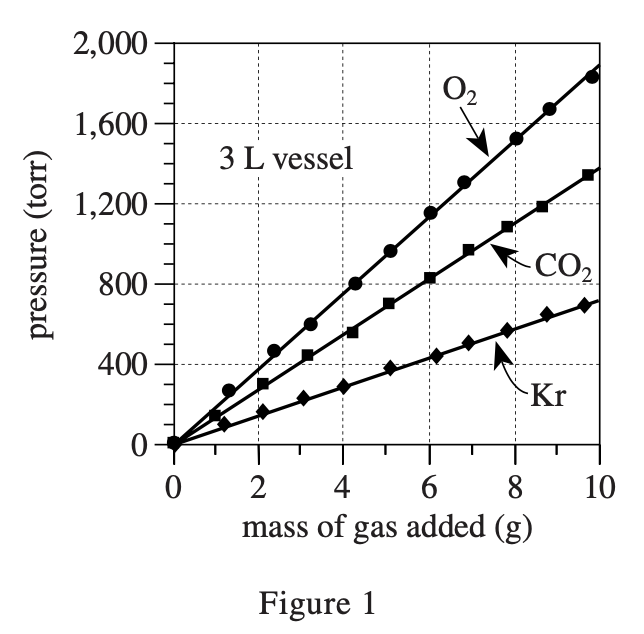
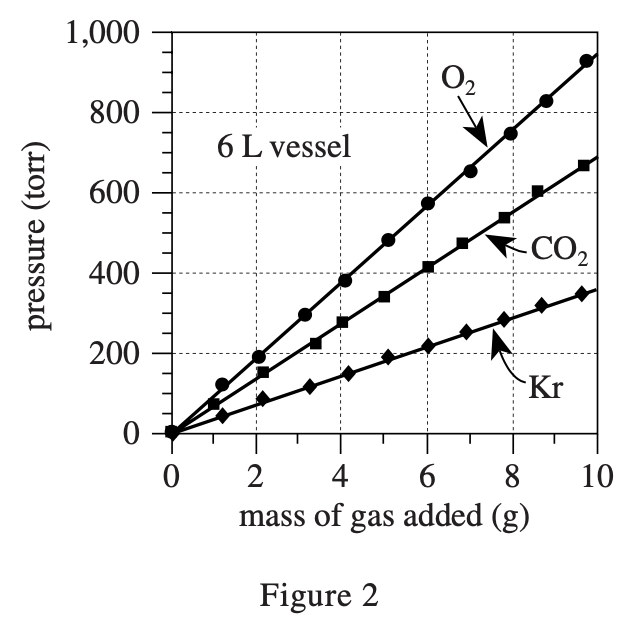
33. Based on Figures 1 and 2, for a given mass of O2 at 22°C, how does the pressure exerted by the O2 in a 6 L vessel compare to the pressure exerted by the O2 in a 3 L vessel? In the 6 L vessel, the O2 pressure will be:
Your Answer is
Correct Answer is A
Explanation
The gas pressure formula in a closed container is P=nRT/V, where n is the number of molecules, R is a constant, T is the temperature, and V is the volume of the container.
So the bigger V is, the smaller P is. So the bigger V is, the smaller P is. It can be calculated by the formula that when V=6, the pressure is half of that when V=3