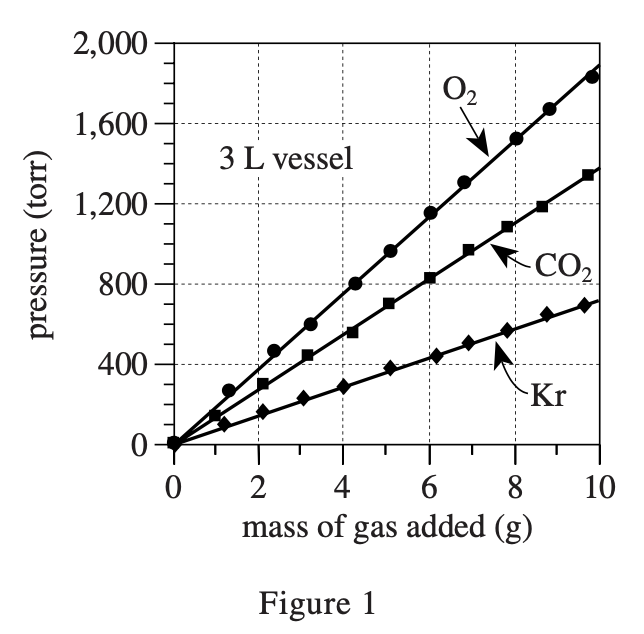
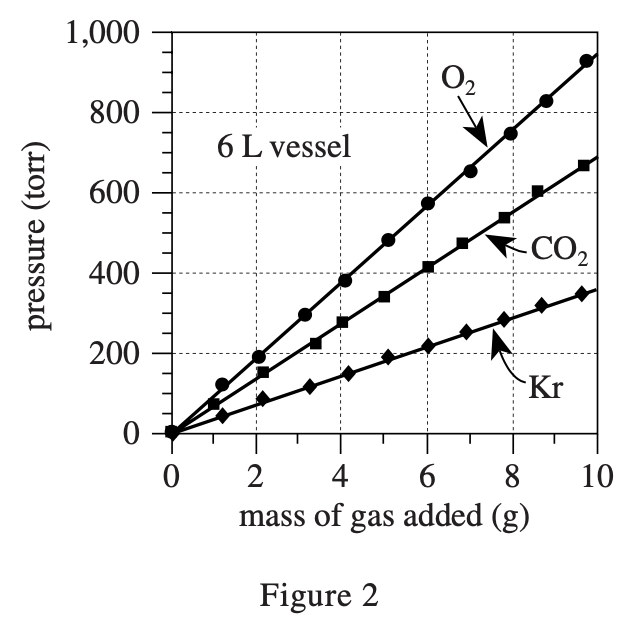
32. Suppose the experiments had been repeated, except with a 5 L vessel. Based on Figures 1 and 2, the pressure exerted by 7 g of CO2 would most likely have been:
Your Answer is
Correct Answer is G
Explanation
The gas pressure formula in a closed container is P=nRT/V, where n is the number of molecules, R is a constant, T is the temperature, and V is the volume of the container.
So the bigger V is, the smaller P is. According to figure 2, when the volume is 6 L and the abscissa is 7, the ordinate corresponding to O2 is about 650. So when the volume is reduced to 5 L, the pressure should increase, but not so much above 1000