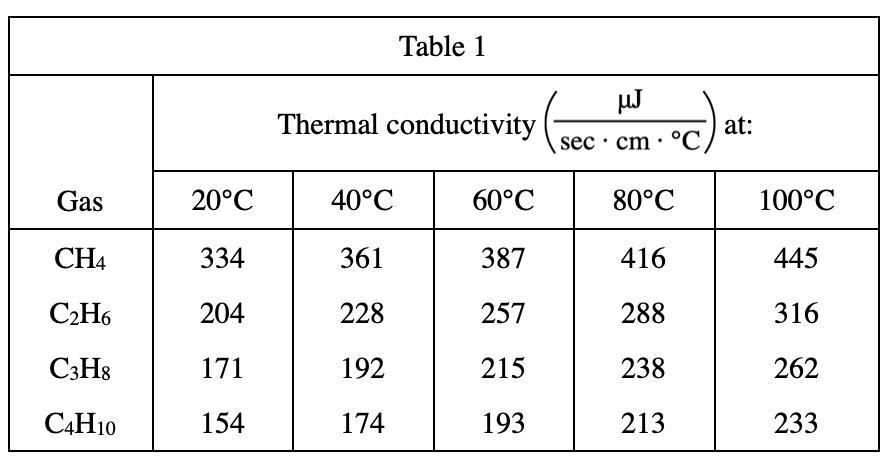
38. Consider the gases carbon dioxide (CO2) and carbon monoxide (CO). Based on Table 1, at a given temperature,which of these gases will have the higher thermal conductivity, and why?
Your Answer is
Correct Answer is H
Explanation
According to table 1, it can be roughly deduced that the larger the molecular mass, the smaller the thermal conductivity. So the thermal conductivity of CO should be greater, because its molecular mass is smaller (the molecular mass of CO is 28, and the molecular mass of CO2 is 44)
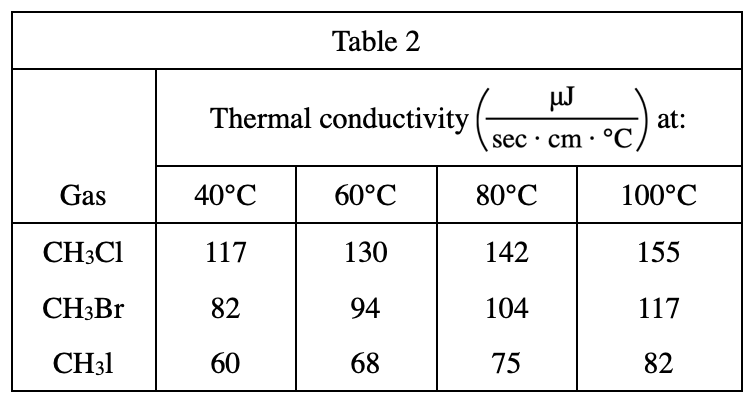
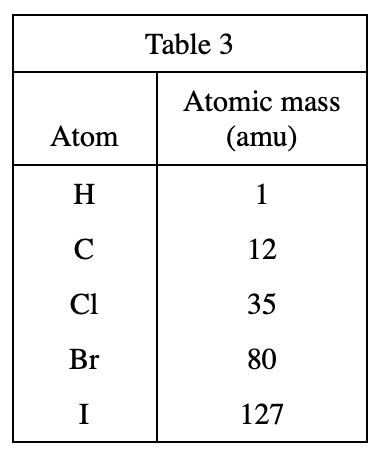
 ; μJ = a unit of energy) of various gases at different temperatures (in °C). Table 3 below lists the masses, in atomic mass units (amu), of the atoms making up the molecules of the gases.
; μJ = a unit of energy) of various gases at different temperatures (in °C). Table 3 below lists the masses, in atomic mass units (amu), of the atoms making up the molecules of the gases.