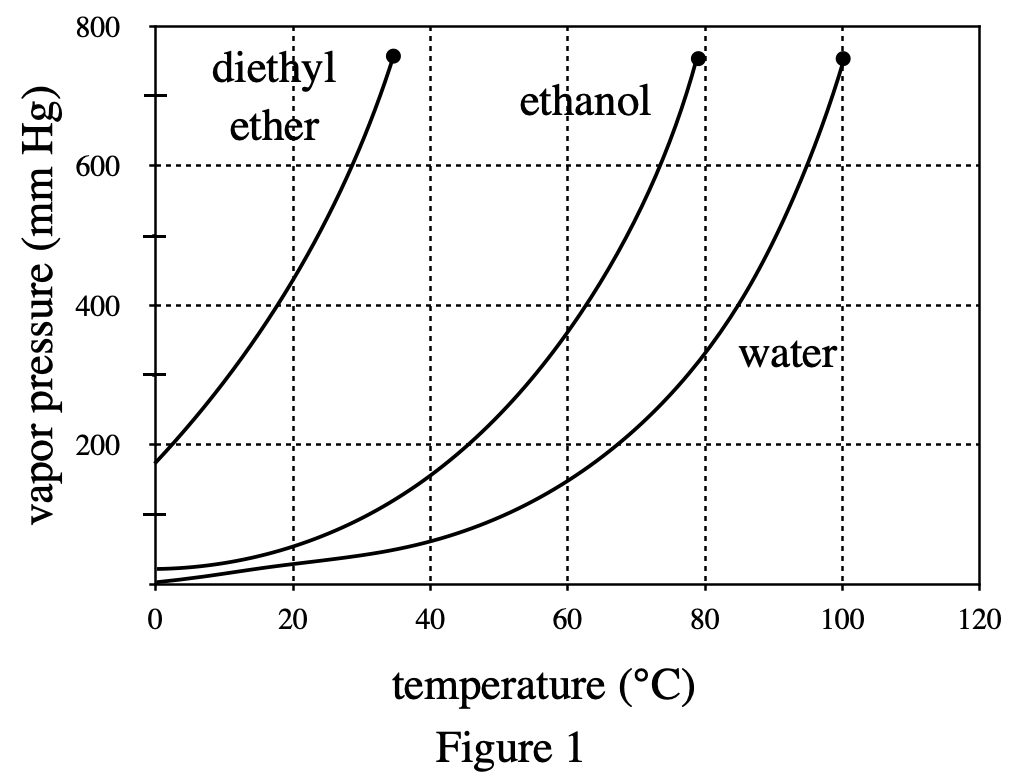
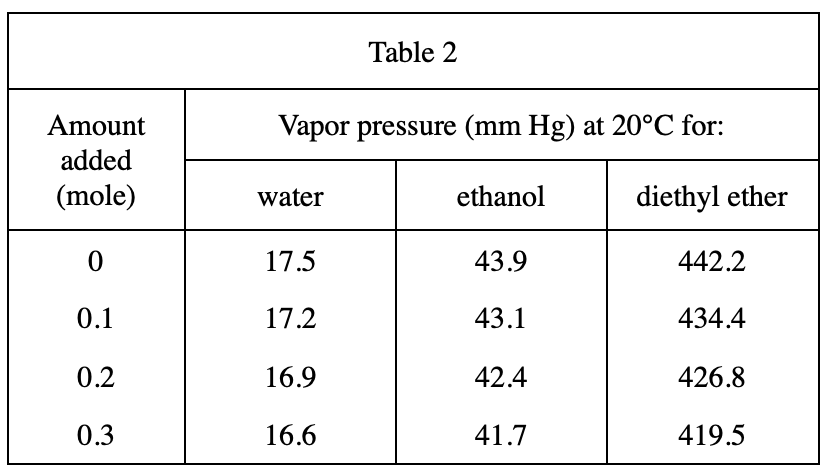
16. Based on the results of Experiment 3, if 0.5 mole of the nonvolatile substance were dissolved in 100 g of diethyl ether, the vapor pressure, in mm Hg, of the solution would most likely be closest to:
Your Answer is
Correct Answer is F
Explanation
See table 2, when the amount added increases, the vapor pressure decreases. Therefore, when the amount added to diethyl ether is 0.5, the vapor pressure should be less than 419.5, excluding H & J; and because when the amount added increases by 0.1, the vapor pressure decreases by about 7, so 0.5 is 0.2 more than 0.3 , the reduced vapor pressure is approximately equal to 7×2=14, 419.5-14=405.5, and option F is closer