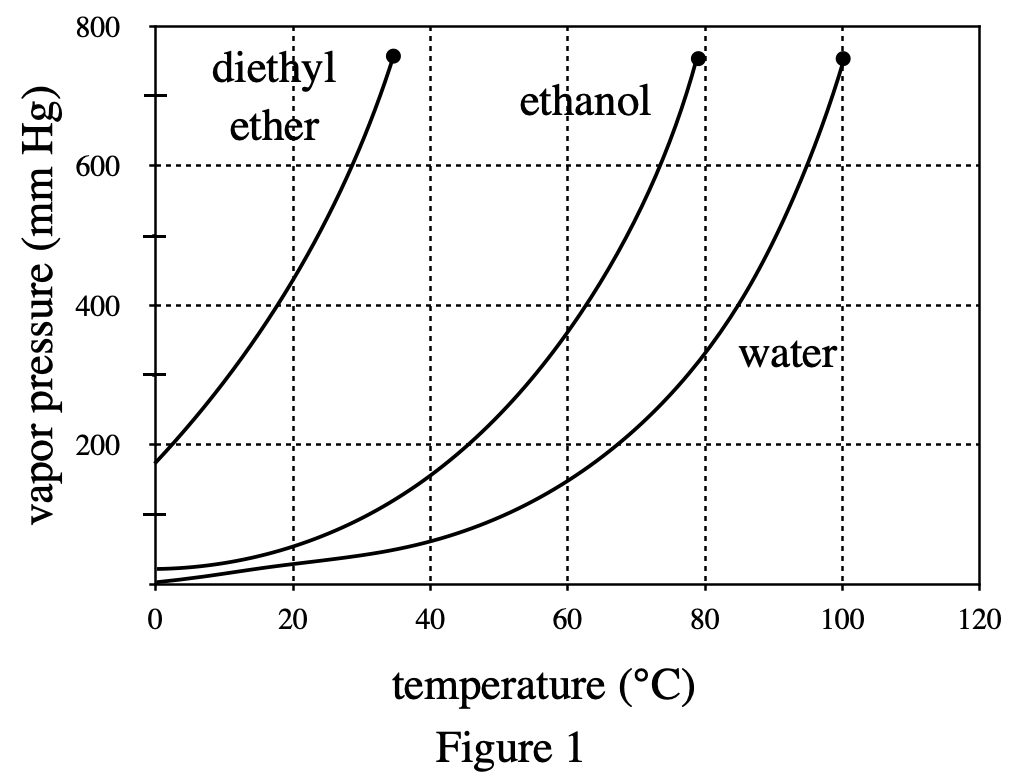
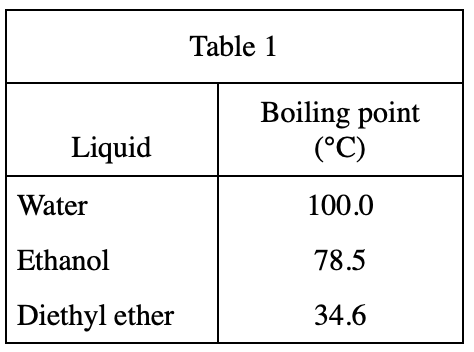
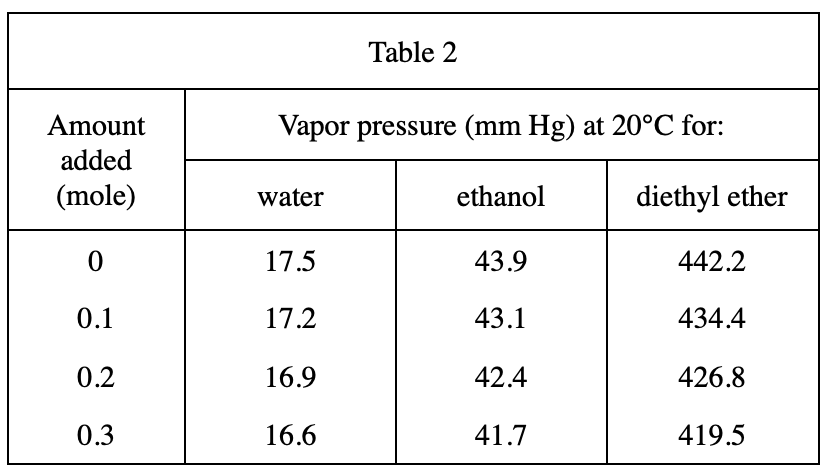
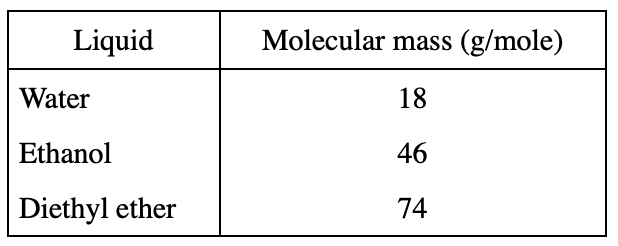
13. A student claimed that liquids with higher molecular masses boil at higher temperatures than liquids with lower molecular masses. Do the results of Experiment 2 and the information in the table below support his claim?
Your Answer is
Correct Answer is B
Explanation
The stem of the question says that the larger the molecular mass, the higher the boiling point. The molecular mass of Water is 18 and its boiling point is 100 degrees. The molecular mass of ethanol is 46. According to table 1, its boiling point is 78 degrees, so the statement in the question is wrong