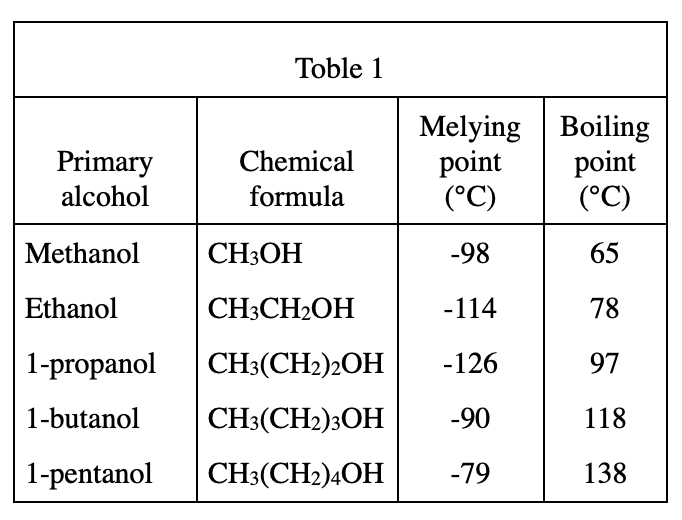
18. The primary alcohol 1-hexanol has the chemical formula CH3(CH2)5OH. Based on Table 1, the boiling point of 1-hexanol is most likely:
Your Answer is
Correct Answer is H
Explanation
Look at table 1. As the molecular weight of alcohols increases, the boiling point becomes higher and higher, so the boiling point of 1-hexanol should be higher than that of 1-pentanol at 138 degrees. For each additional C atom, the boiling point increases by about 20 degrees (except for methanol-ethanol). So the boiling point of 1-hexanol should be around 138+20=158. The closest should be option H