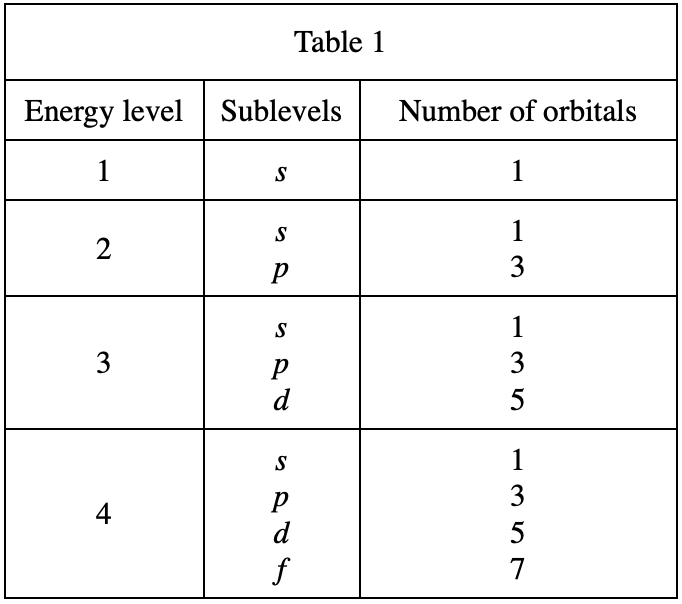
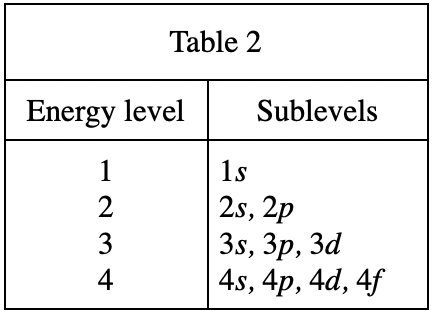
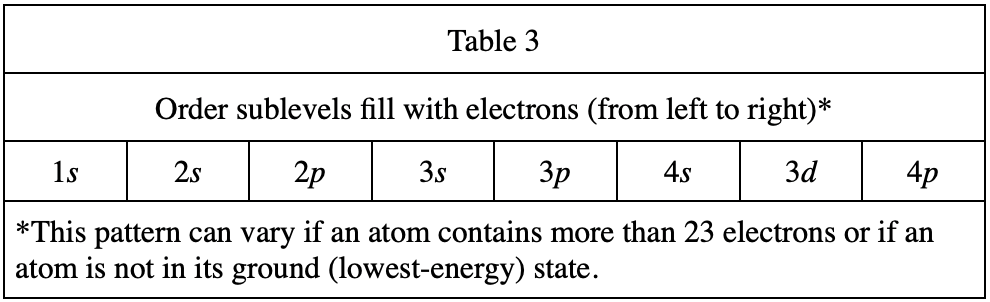
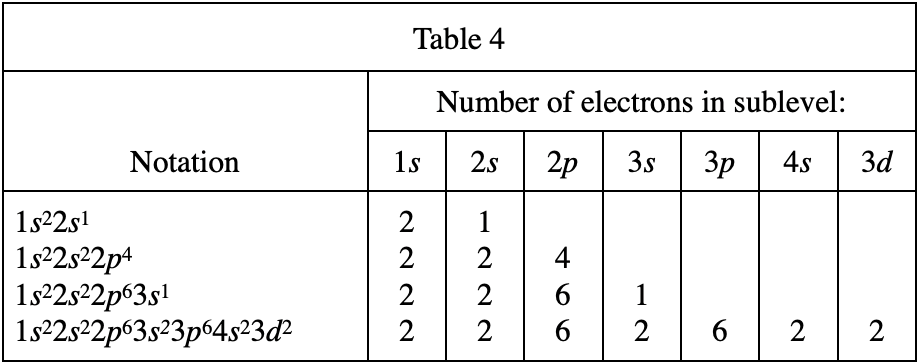
32. As the number of electrons in a group of ground state atoms increases from 3 to 13, the number of electrons in Sublevel:
Your Answer is
Correct Answer is G
Explanation
According to table 4, when there are 3 electrons, it should be 1s22s1;
When there are 13 electrons, it should be It is 1s22s22p63s23p1, so 1s this The number of electrons on the sublevel remains unchanged