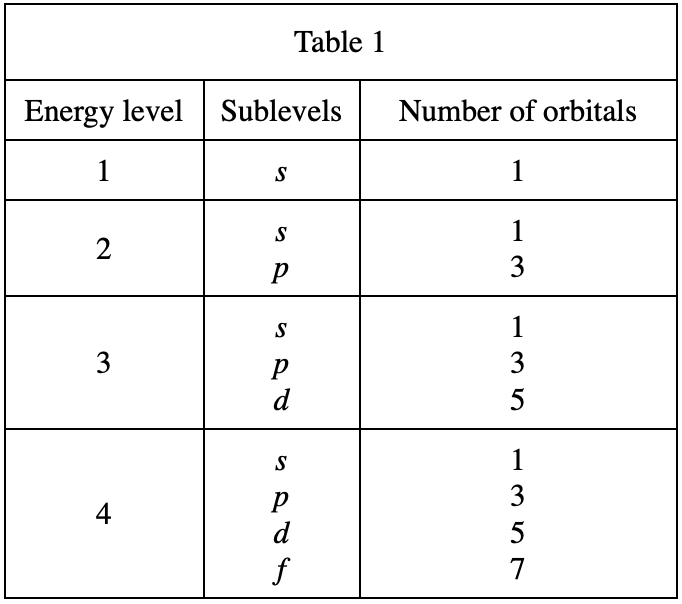
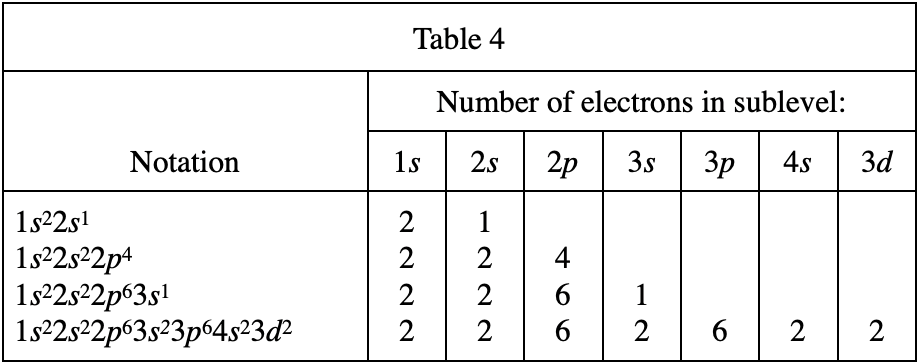
31. If an atom in its ground state has 15 electrons, how many electrons are in the 3p sublevel?
Your Answer is
Correct Answer is C
Explanation
From table 4, it can be seen that there are at most 2 electrons on 1s, at most two electrons on 2s, at most 6 electrons on 2p, and at most 2 electrons on 3s, then 2+2+6+2=12 , so when an atom has a total of 15 electrons, the 3p sublevel should reach 15-12=3 electrons