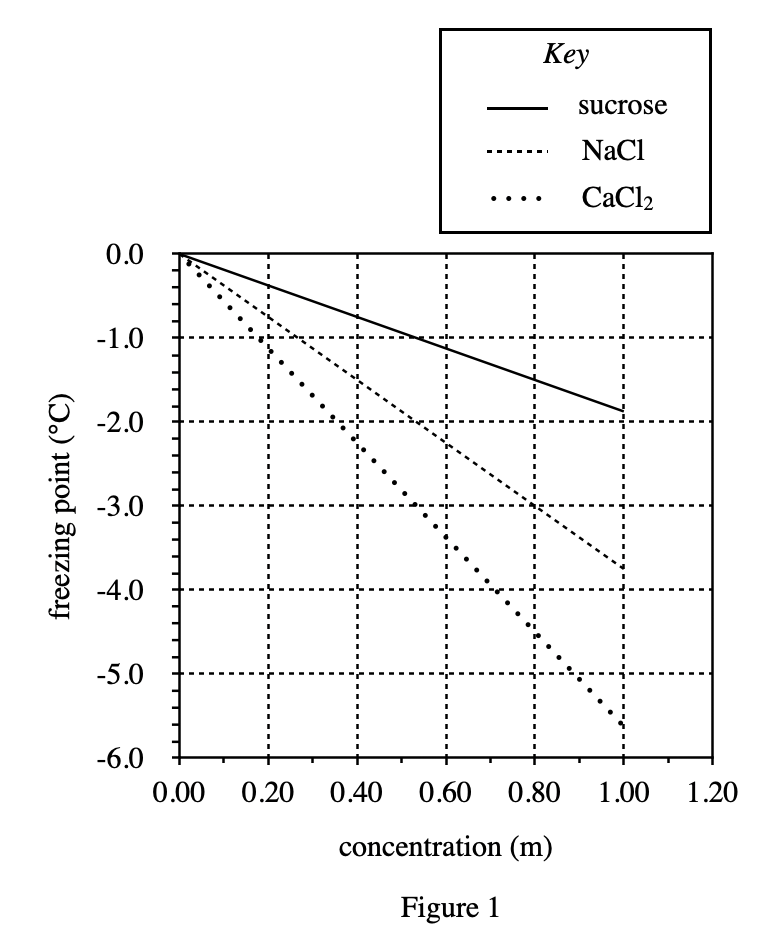
16. Based on Figure 1, as the mass of sucrose dissolved in a given amount of solvent increases, the freezing point of the solution will:
Your Answer is
Correct Answer is H
Explanation
The increase in the mass of Sucrose is actually equivalent to the increase in concentration. Look at figure 1, as the abscissa concentration increases, the ordinate freezing point decreases in a straight line