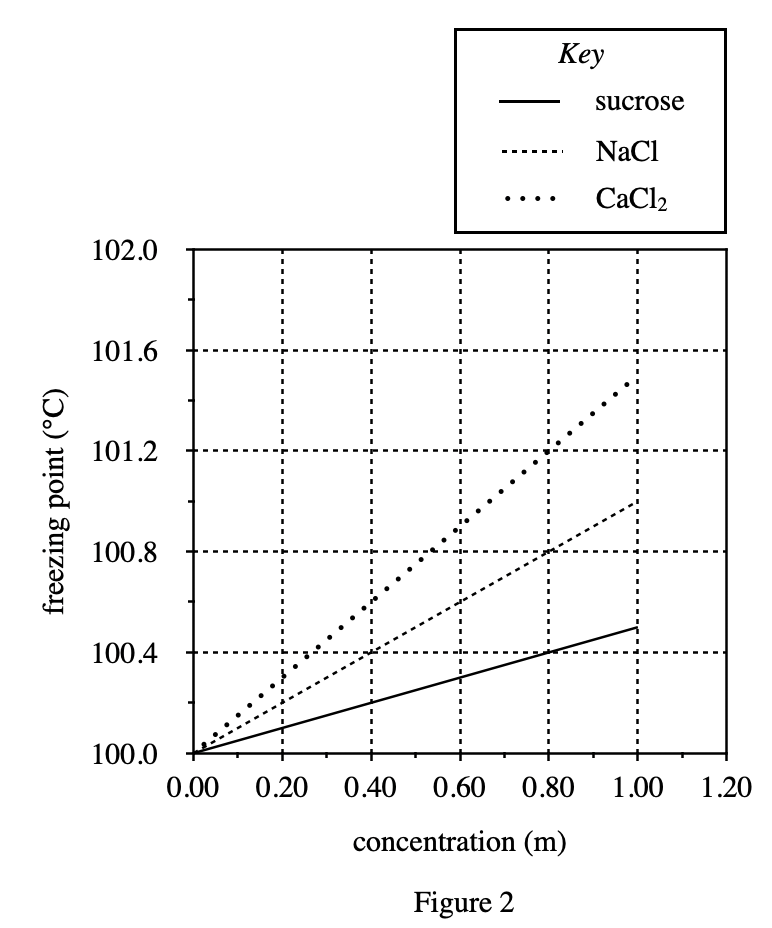
13. Based on the trends in Figure 2, which of the following aqueous solutions would have the highest boiling point ?
Your Answer is
Correct Answer is A
Explanation
Put value questions. See figure 2, whenever the concentration concentration of CaCl2 solution increases by 0.8, the boiling point increases by 1.2 °C;
When the concentration concentration of NaCl solution increases by 0.8, the boiling point increases approximately 0.8 °C;
When the concentration of sucrose solution increases by 0.8, the boiling point increases by approximately 0.4 °C.
Therefore, the corresponding boiling point of 1.20 m CaCl2 solution is about 101.8°C, the corresponding boiling point of 1.4 m NaCl solution is about 101.4°C, and the corresponding boiling point of 1.6 m sucrose solution is about 100.8 °C, 1.8 m sucrose solution corresponds to a boiling point of about 100.9°C, the highest boiling point is 1.2 m CaCl2