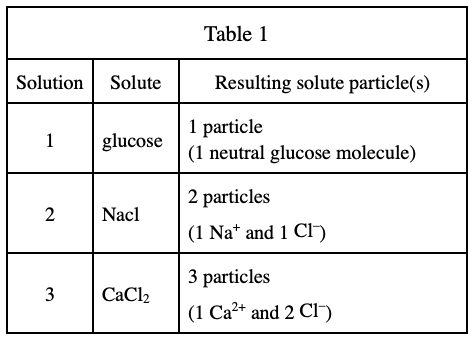
38. When table sugar is dissolved in pure H2O at 25℃, the vapor pressure at equilibrium is lower than that of pure H2O at 25℃. Because dissolved table sugar molecules are neutral, this observation contradicts the explanation of:
Your Answer is
Correct Answer is F
Explanation
Look at the last two lines of Student 1, Neutral solute particles do not affect the vapor pressure of a liquid, so the statement in the question stem contradicts Student 1