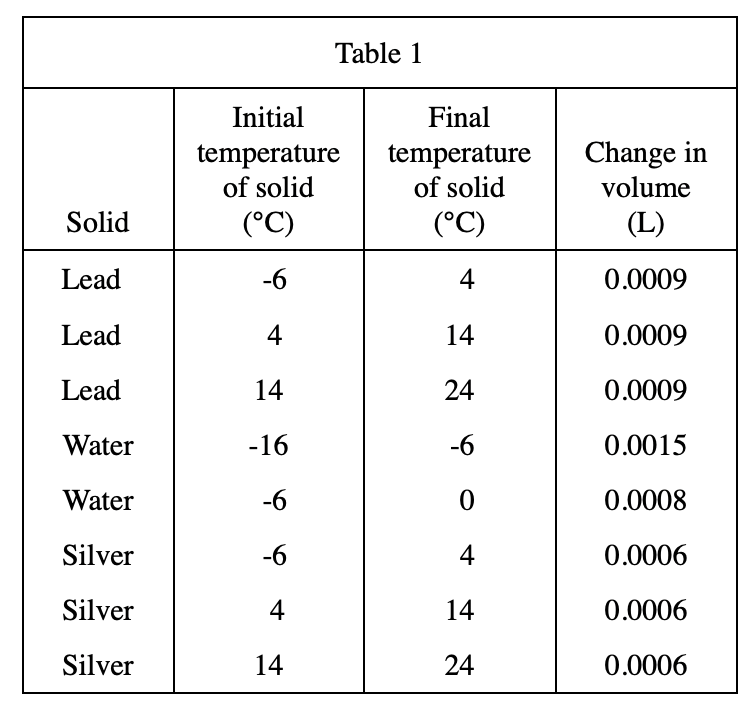
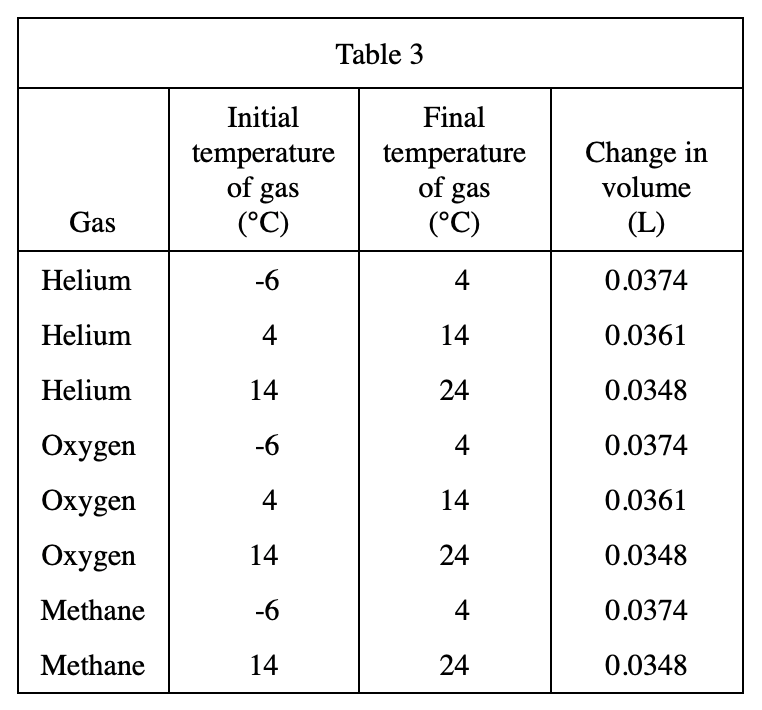
23. Based on Table 3, if 1.0000 L of helium at 24°C is heated to 34°C at constant pressure, the change in volume will most likely be closest to which of the following?
Your Answer is
Correct Answer is A
Explanation
It can be seen from table 3 that the higher the initial temperature of helium, the smaller the volume change after every 10°C increase, so when helium is heated from 24°C to 34°C, the volume change should be less than 0.0348 L