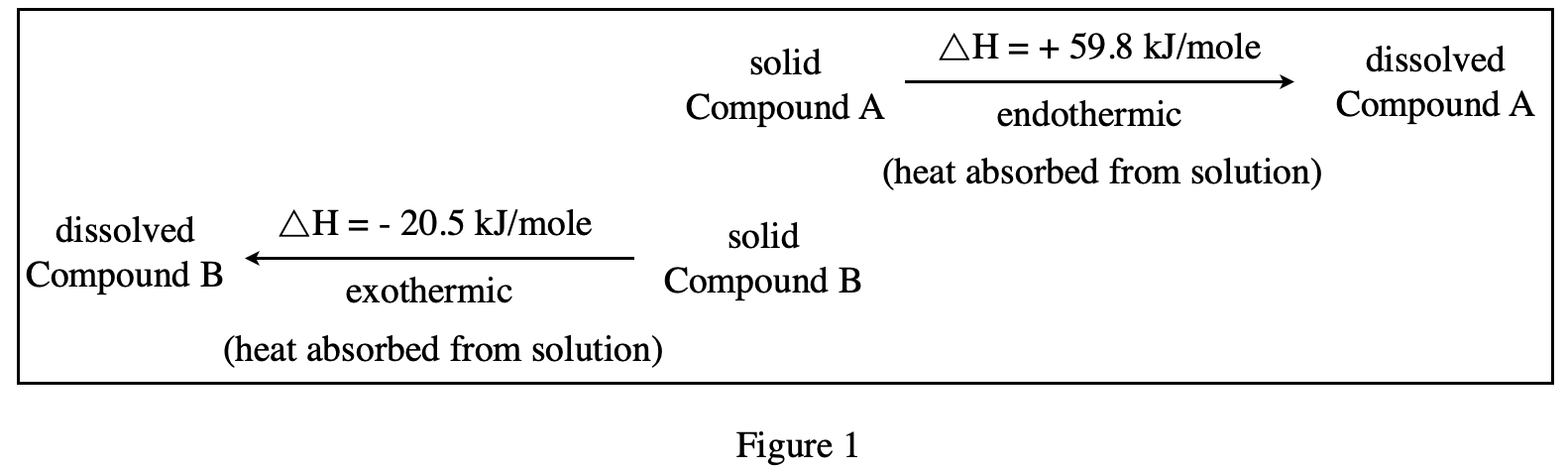
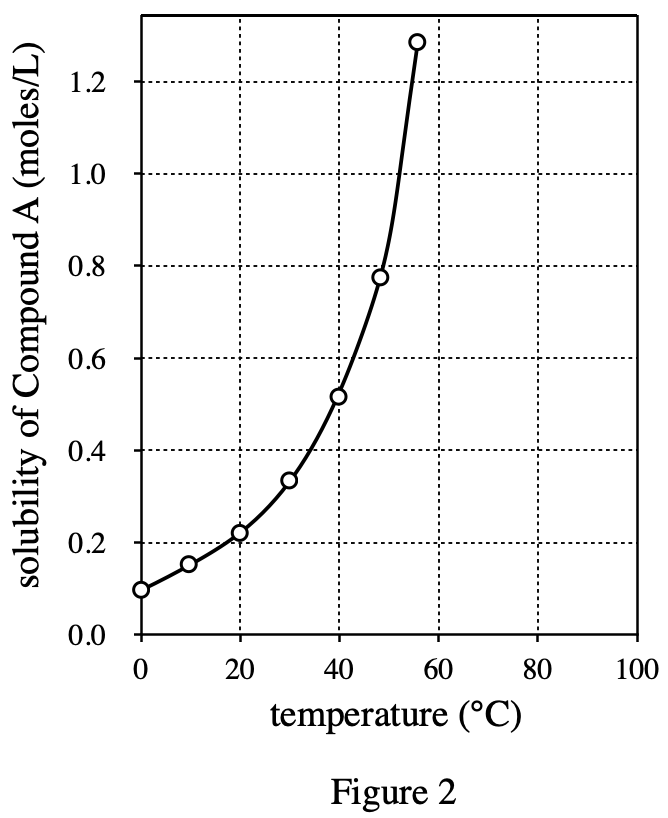
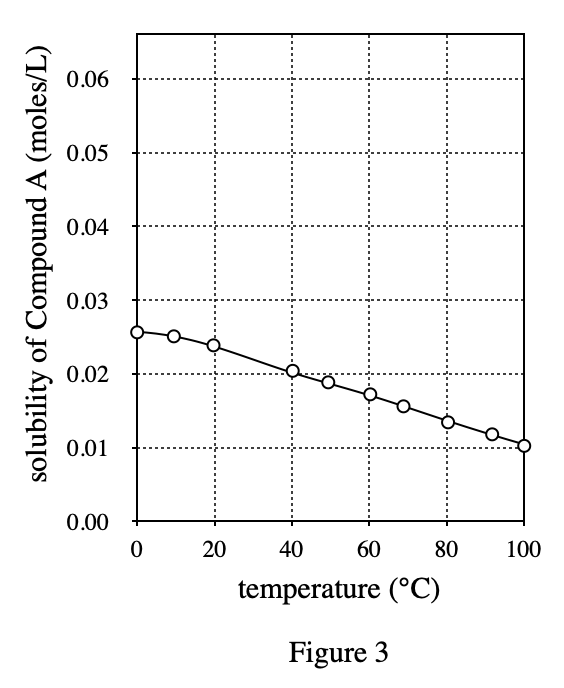
35. Ammonium nitrate is a compound that has a △H of +25.7 kJ/mole when it dissolves in water at 25°C. Based on the data in Figure 1, if l mole each of ammonium nitrate and Compound A are dissolved separately in equal amounts of water at 25℃, how will the transfer of heat compare?
Your Answer is
Correct Answer is A
Explanation
According to figure 1, the ΔH of Compound A=+59.8 kJ/mole is higher than the ΔH of ammonium nitrate in the question, indicating that Compound A will absorb more heat, while ammonium nitrate absorbs relatively less heat