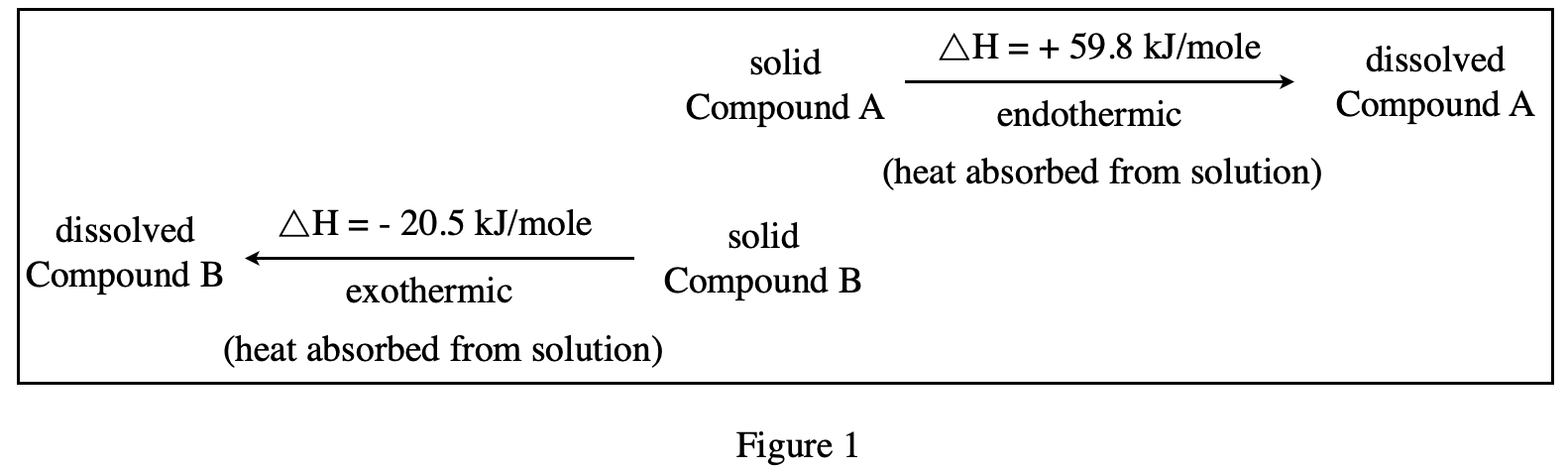
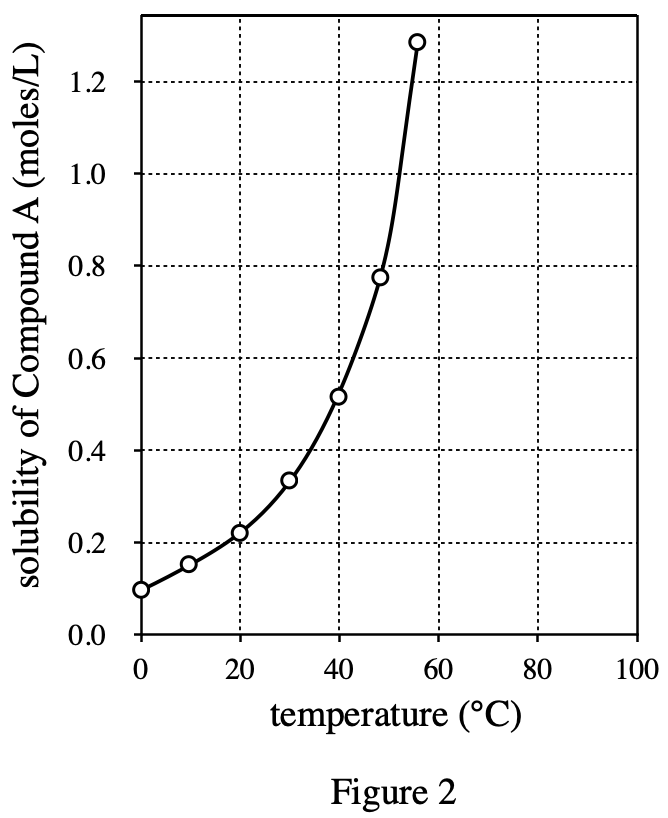
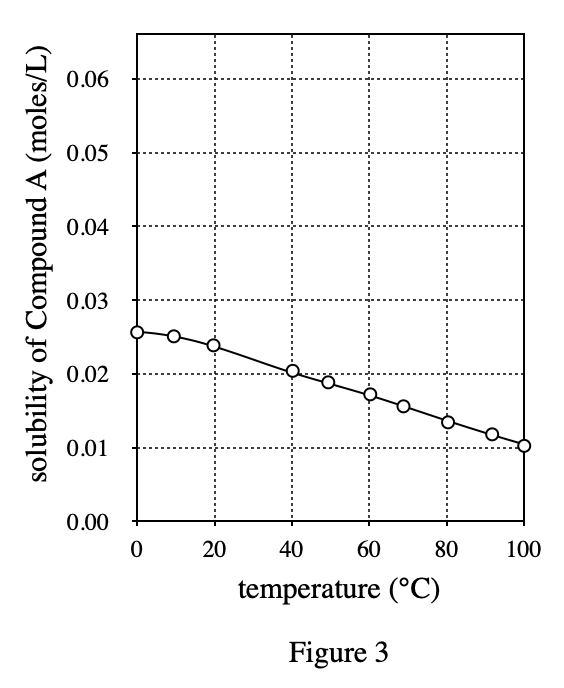
34. A chemist added either Compound A or Compound B to water at 25℃. The temperature of the solution decreased until the solution began to freeze. Based on the data in Figure 1, which compound did he most likely add to the water?
Your Answer is
Correct Answer is F
Explanation
After adding compound, the temperature of the solution drops, indicating that the solute is endothermic (ΔH is a positive number), so according to figure 1, it is known that Compound A should be added