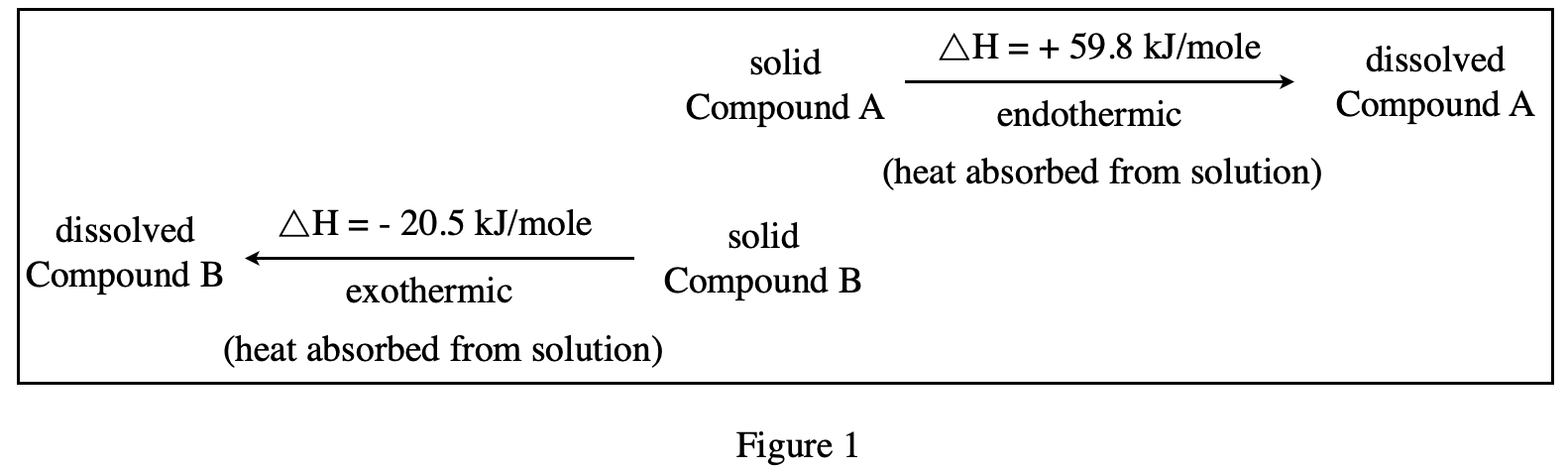
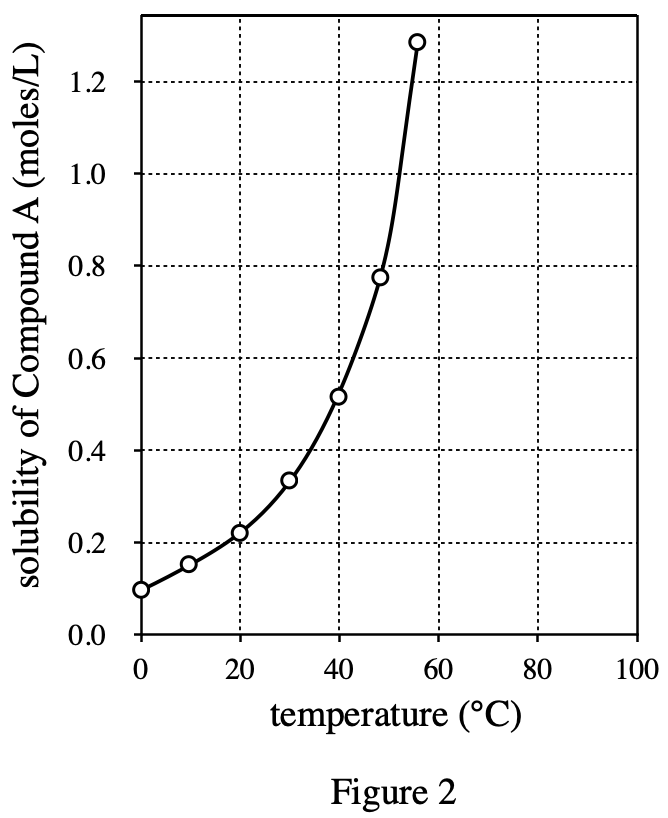
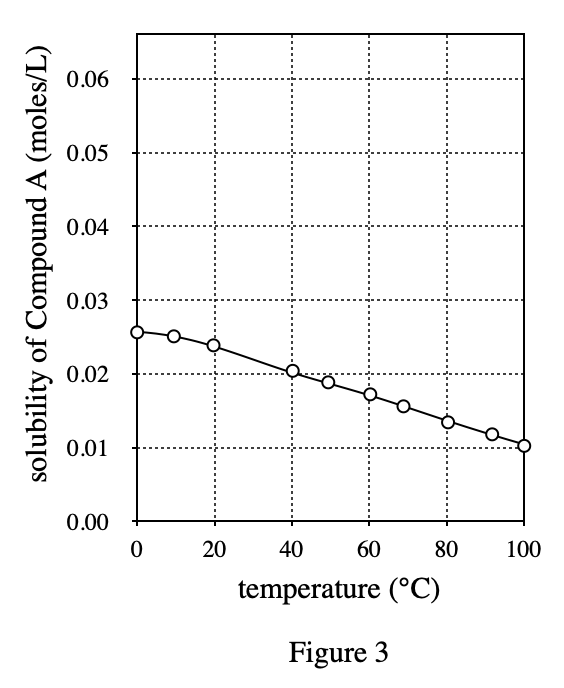
32. A solution is made that has concentration a of 1 mole/L of Compound A and 0.5 mole/L of Compound B. According to the passage, the number of molecules of Compound A in the solution is:
Your Answer is
Correct Answer is H
Explanation
According to the question, in 1L of solution, Compound A has 1 mole, and Compound B has 0.5 mole, so the number of molecules of A is twice that of B