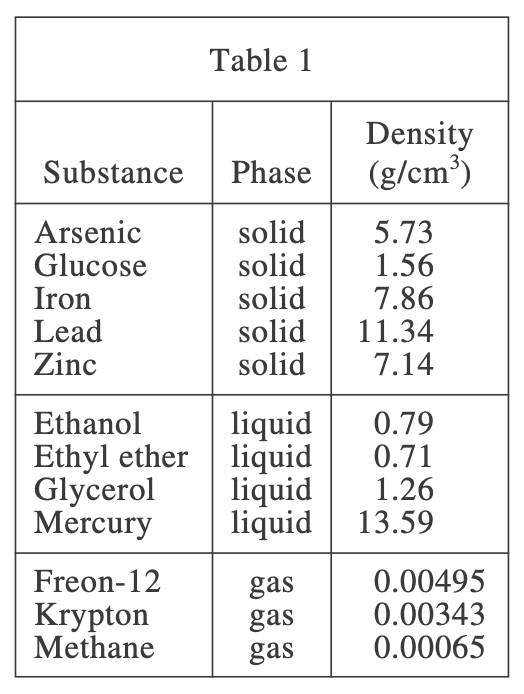
28. Equal amounts of ethyl ether, mercury, and water (density = 0.9971 g/cm3) at 25°C are poured into a single beaker. Three distinct layers of liquid form in the beaker. Based on the data in Table 1, which of the following diagrams represents the order, from top to bottom, of the liquids in the beaker?
Your Answer is
Correct Answer is F
Explanation
General knowledge questions.
Those with high density sink to the bottom, and those with low density float up. Looking at table 1, the density of mercury is 13.59 g/cm3, the density of ethyl ether is 0.71 g/cm3, and the density of water is 0.9971 g/cm 3, so ethyl ether has the lowest density and is on the top layer; mercury has the highest density and is on the bottom layer. F option meets