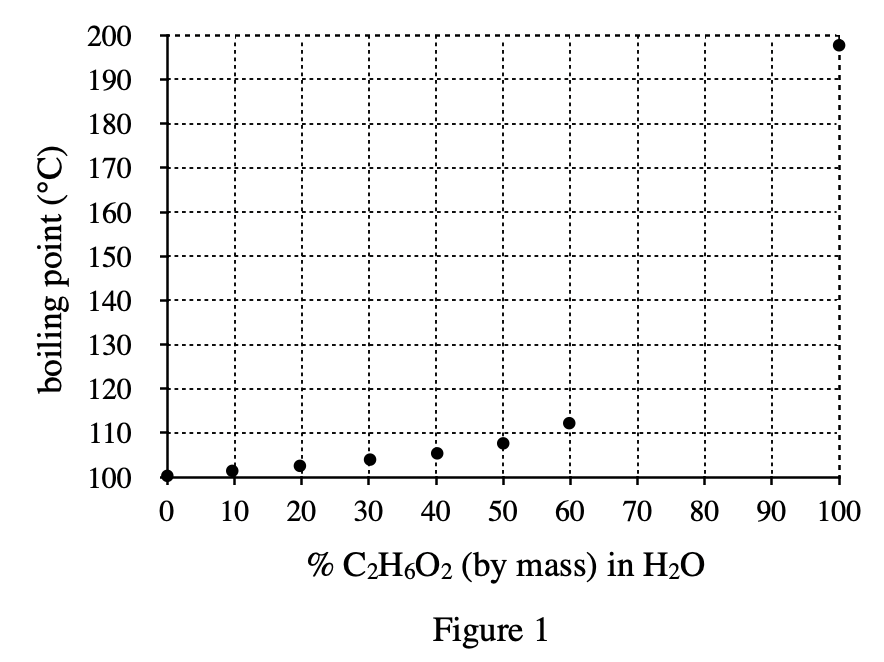
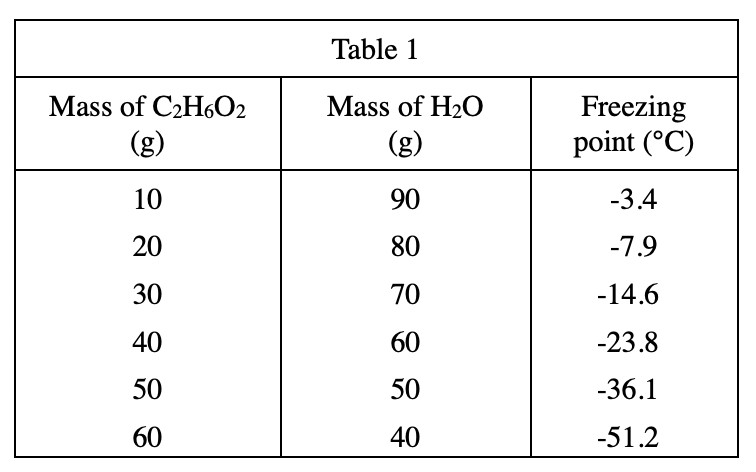
22. A student repeating Experiment 3 placed an aqueous mixture containing 65% C2H6O2 in the low-temperature bath. She did not observe the formation of any crystals. Which of the following statements best explains this observation?
Your Answer is
Correct Answer is G
Explanation
According to table 1, it can be seen that the more mass of C2H6O2 in the solution, the lower the freezing point.
The middle school students use 65% concentration of C2H6O2, so the freezing point should be lower than -51.2°C at this time. The solution does not freeze, indicating that the temperature of the bath is higher than the freezing point