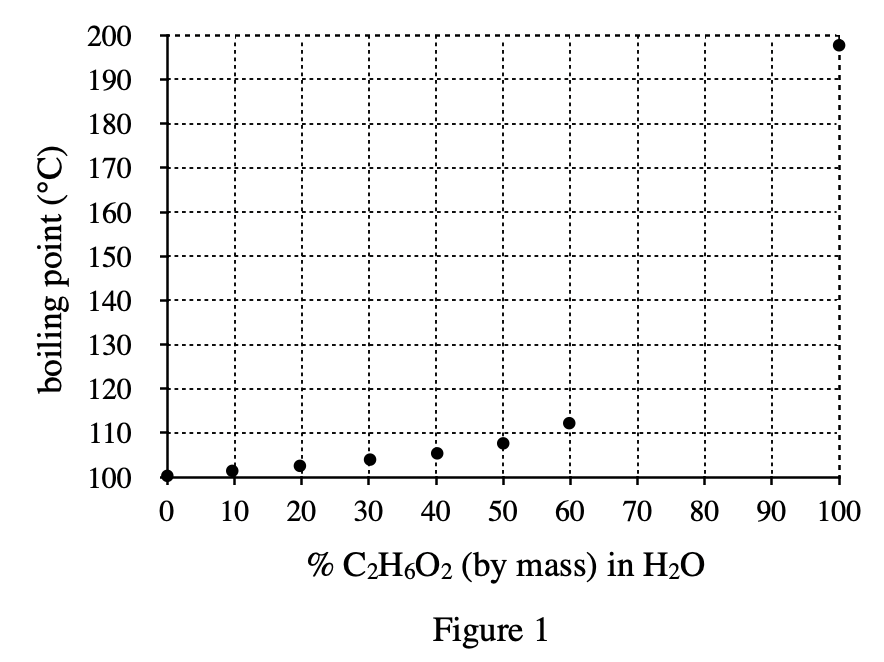
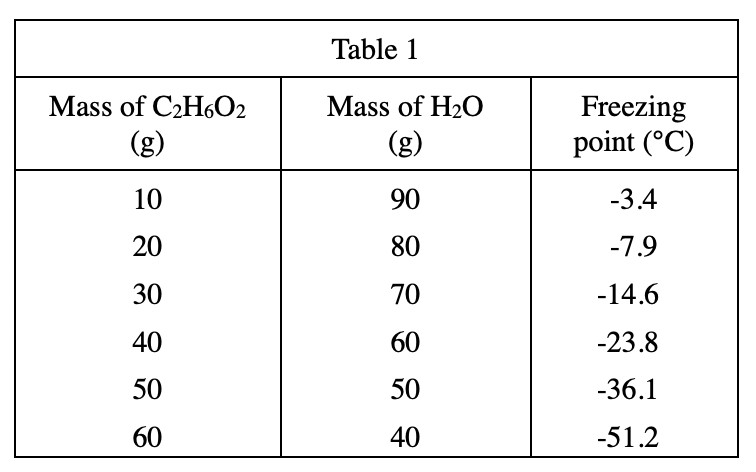
21. Based on the results of Experiment 1, the mass of 10.00 mL of which of the following aqueous solutions would be the greatest?
Your Answer is
Correct Answer is D
Explanation
Look at the formula density=mass/volume given in Experiment 1, introduce mass=density×volume, and give a fixed volume of 10.00 mL in the question stem, so the larger the density, the larger the mass should be.
According to the text below the formula, it can be seen that the higher the concentration of C2H6O2, the greater the density of the solution, so you should choose the one with the highest concentration