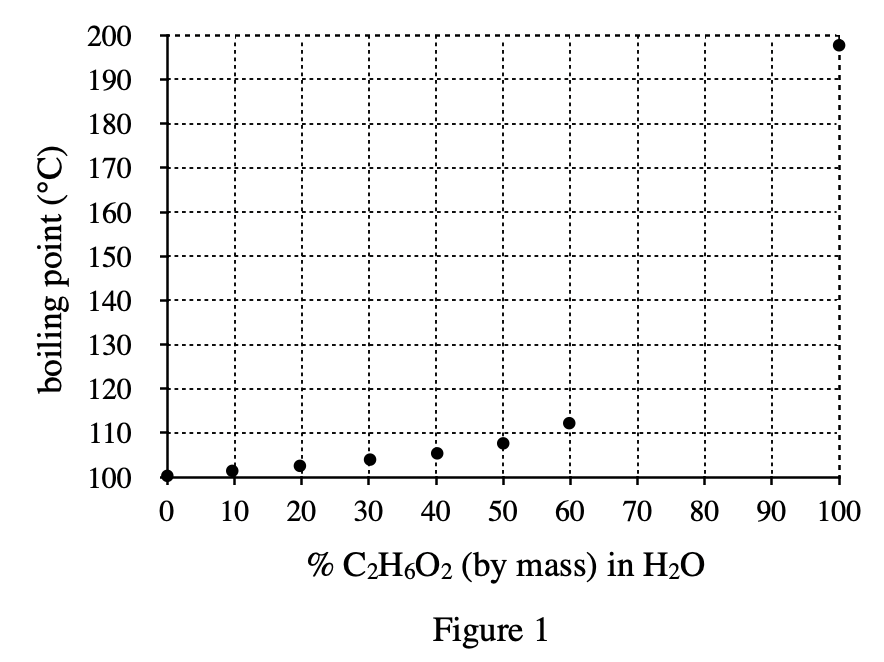
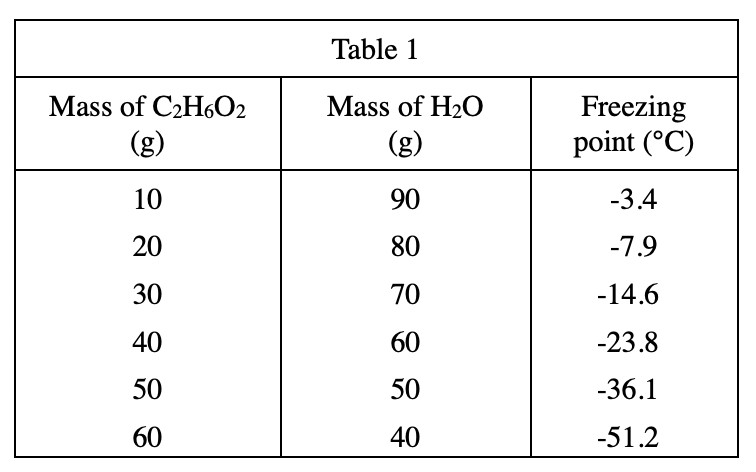
20. Where the students live, the lowest temperature during the past year was -31°C. Based on the results of Experiment 3, which of the following percents, by mass, of C2H6O2 in H2O would have been necessary to be sure that the mixtures in car radiators in this area did not freeze?
Your Answer is
Correct Answer is J
Explanation
Look at table 1, when the Mass of C2H6O2 is 50 g, the mass of H2O is also 50 g, namely C When the concentration of 2H6O2 is 50%, the freezing point is -36.1°C;
题目The lowest temperature in is -31°C, so a 50% solution will not freeze at this temperature