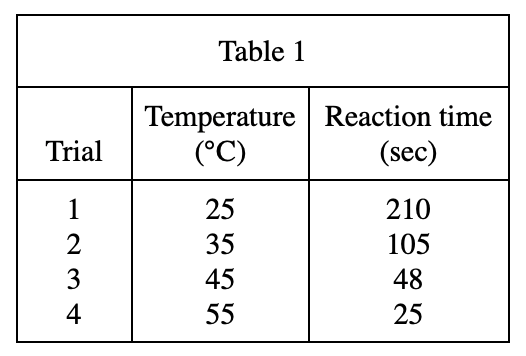
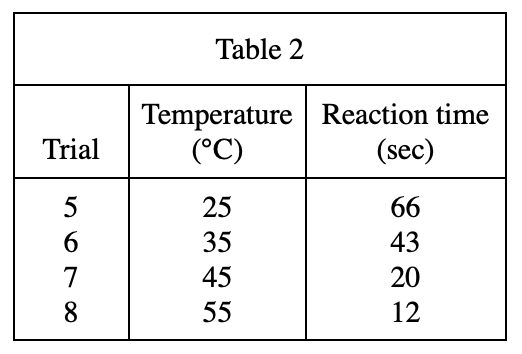
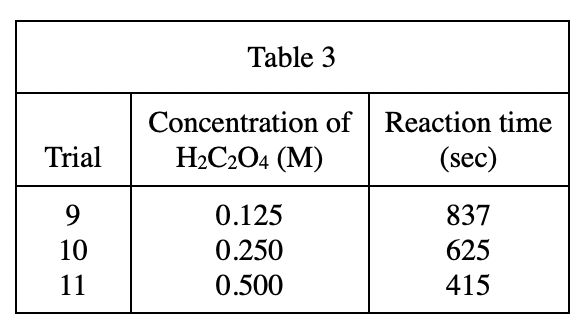
13. A student repeating Trial 9 unknowingly used a graduated cylinder that already contained 1.0 mL of H2O to measure the H2C2O4 solution. Based on the results of Experiment 3, the reaction time that she measured was most likely:
Your Answer is
Correct Answer is D
Explanation
Because there is still 1 mL of water, the Concentration of H2C2O4 must be less than 0.125 in trail 9, so the reaction time must be longer than 837 sec (as can be seen from table 3, the lower the concentration, the longer the reaction time)