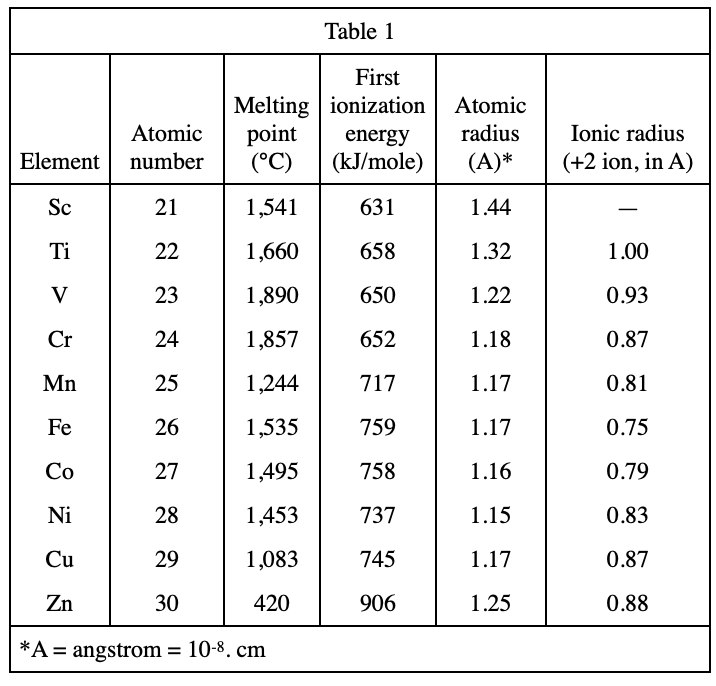
23. The second ionization energy is the energy needed to remove one electron from the +1 ion. For Zn, this energy is 1, 733 kJ/mole. How much energy is required to change the neutral Zn atom into its +2 ion?
Your Answer is
Correct Answer is D
Explanation
The energy required to take away an electron is the first ionization energy, and then some energy is needed to take an electron away again, which is the second ionization energy. The sum of the two energies is the total energy required to take away the two outer electrons, choose D