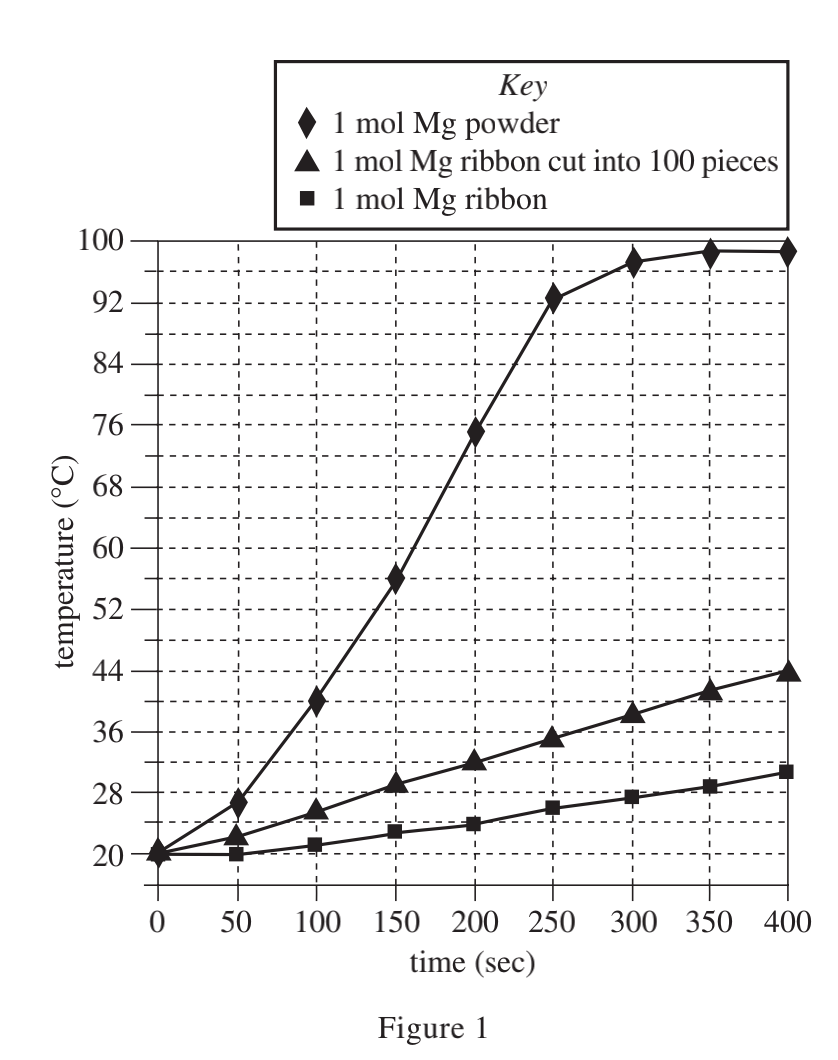
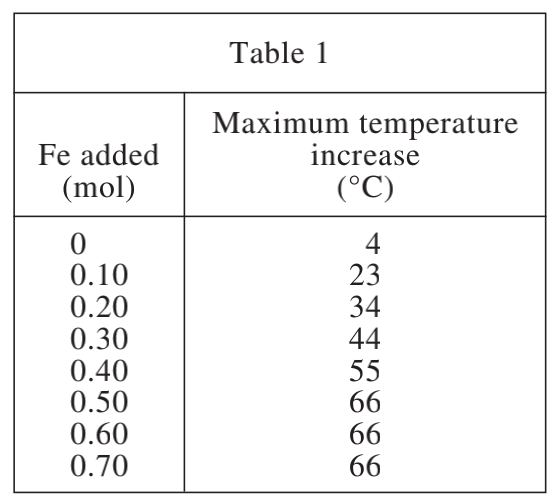
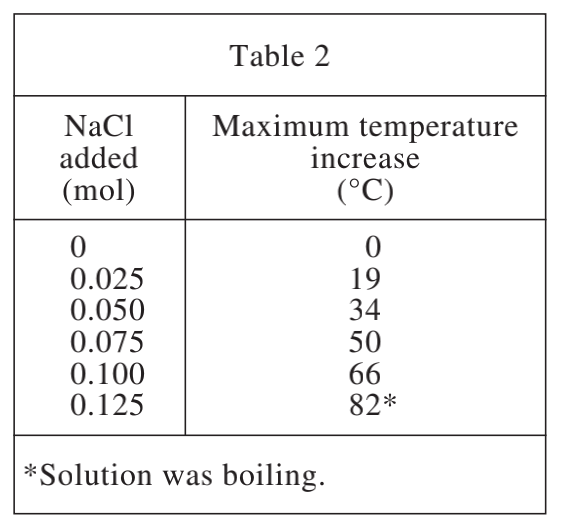
35. It has been observed that as Mg and H2O react, the Mg(OH)2 (magnesium hydroxide) that is produced forms an unreactive coating on the Mg surface. Which of the following models for why NaCl speeds up the reaction is most consistent with this observation and the results of the experiments?
Your Answer is
Correct Answer is D
Explanation
Mg(OH)2 on the surface of Mg will hinder the further exothermic reaction. Comparing table 1 and table 2, it is found that after adding NaCl, the temperature rises higher, indicating that NaCl can remove Mg(OH)2