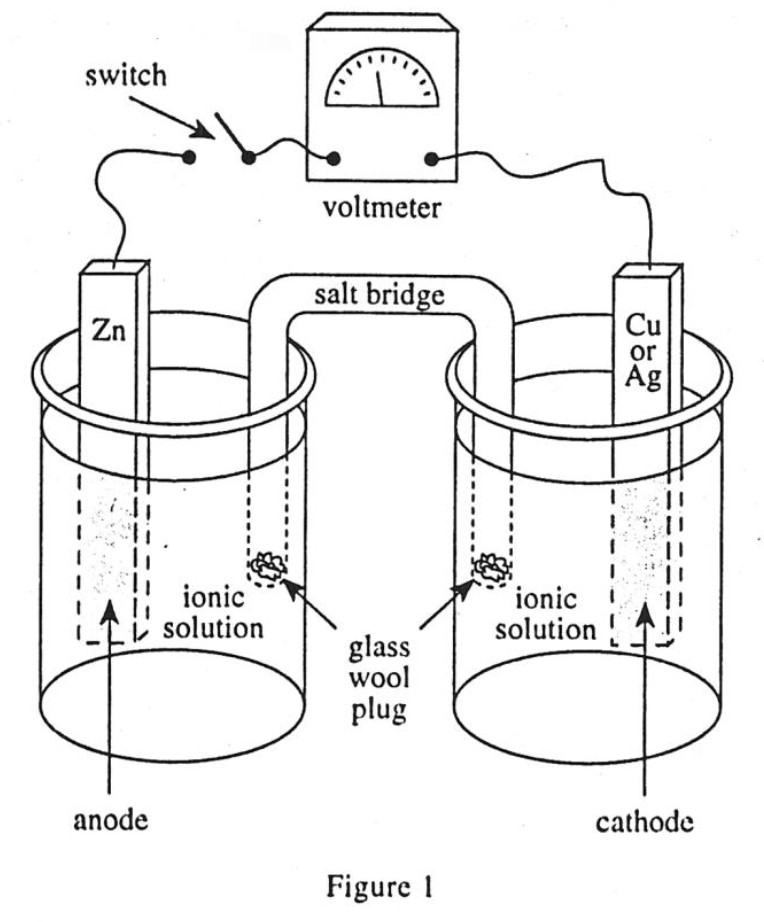
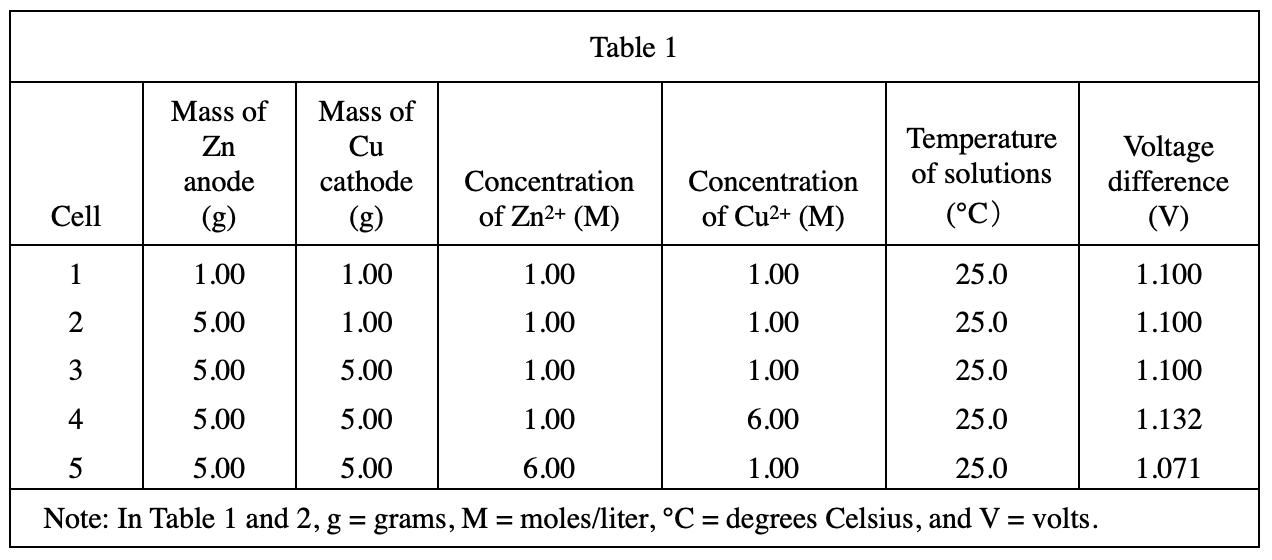
13. Based on the data in Table 1, if an electrochemical cell is constructed with a 5.00 g Zn anode placed in 1.00 M Zn2+ and a 5.00 g Cu cathode placed in 1.00 M Cu2+ at 25°C, what voltage difference is produced?
Your Answer is
Correct Answer is B
Explanation
According to the description in the question stem, it should correspond to Cell 3 in table 1, and the voltage difference is 1100