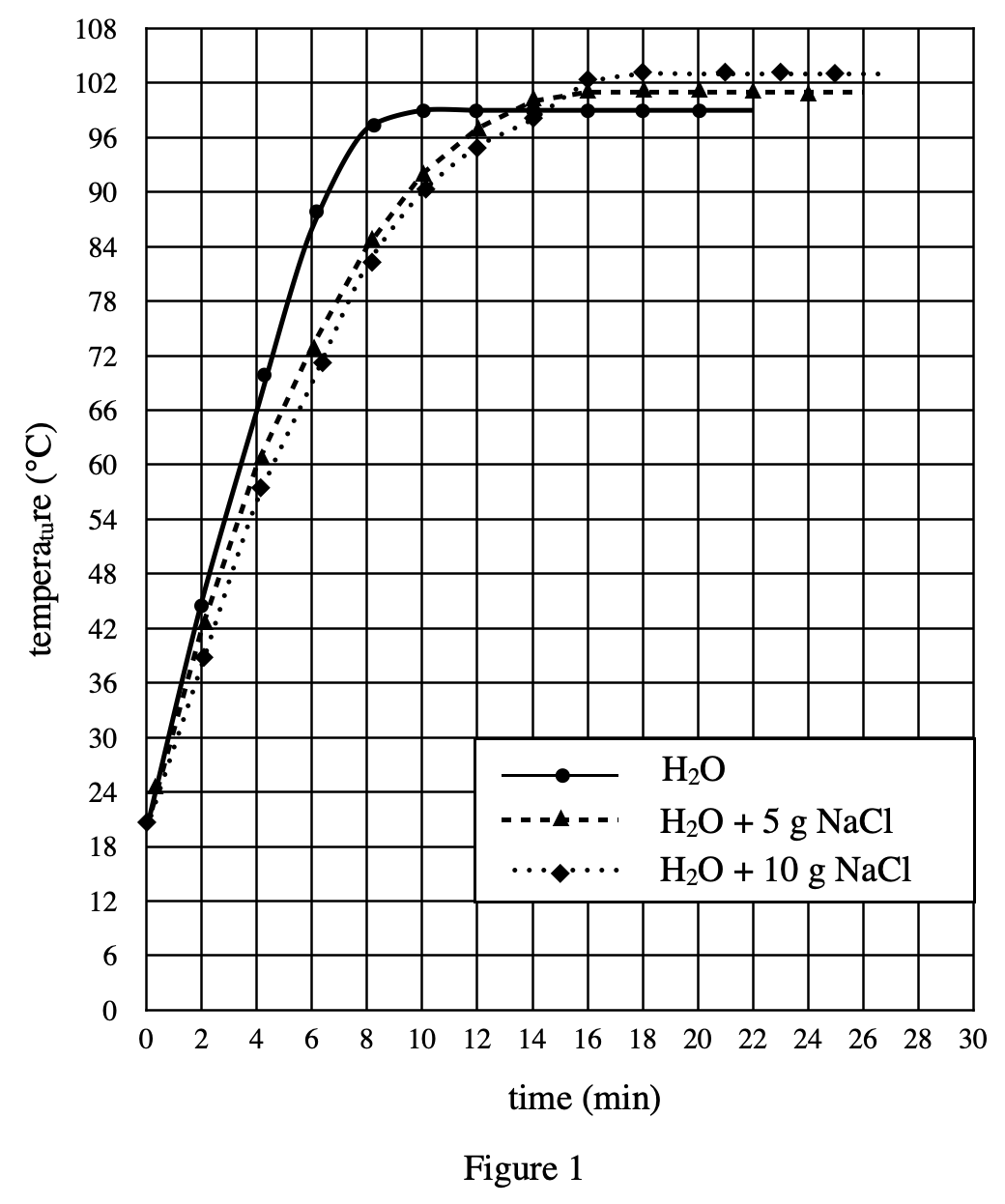
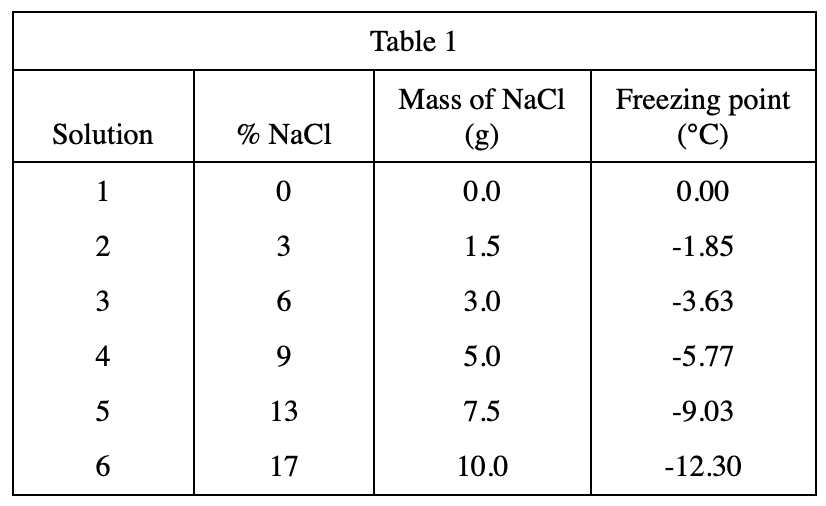
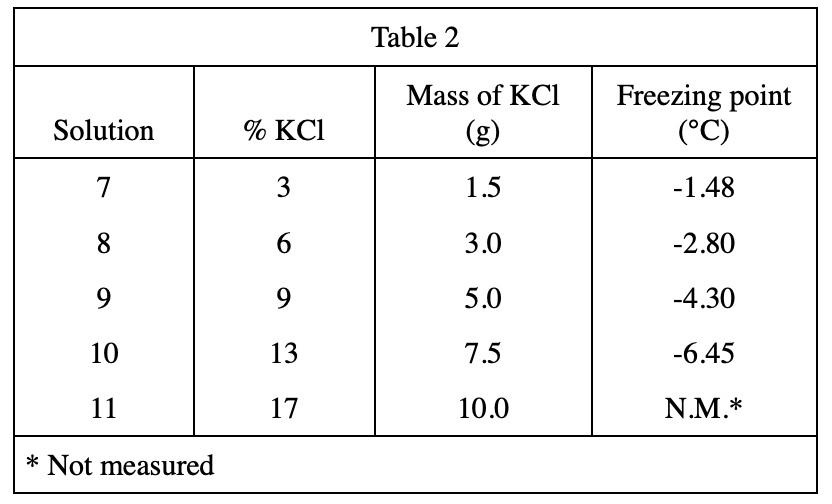
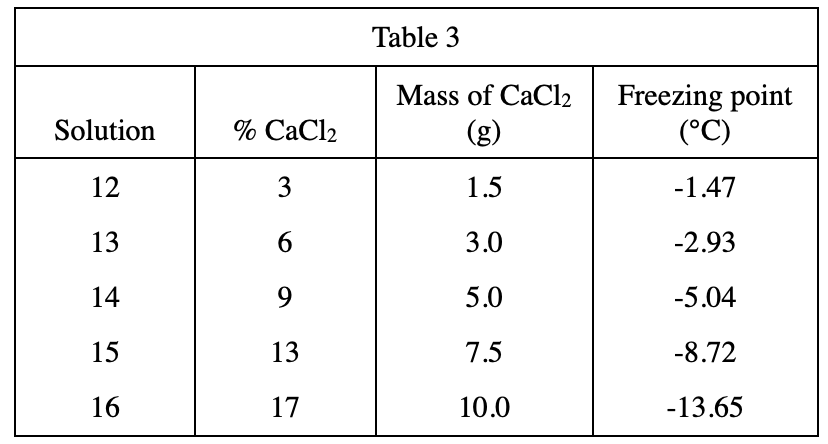
19. Which of the following procedures would most likely raise the freezing point of all of the solutions measured in Experiment 3 ?
Your Answer is
Correct Answer is C
Explanation
It can be seen from table 3 that the lower the %CaCl2, the higher the freezing point. So adding water to the solution will reduce the %CaCl2, which can increase the freezing point