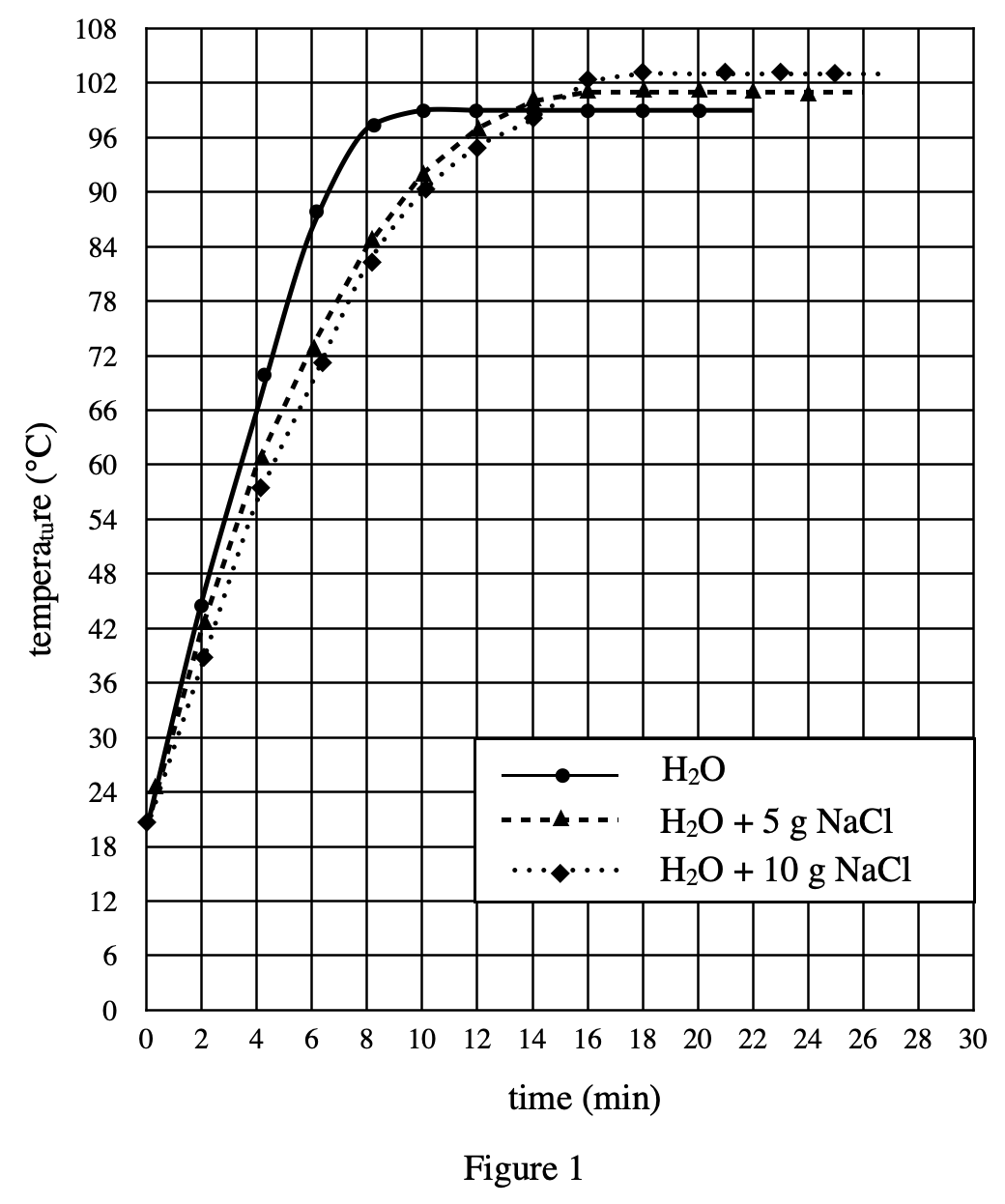
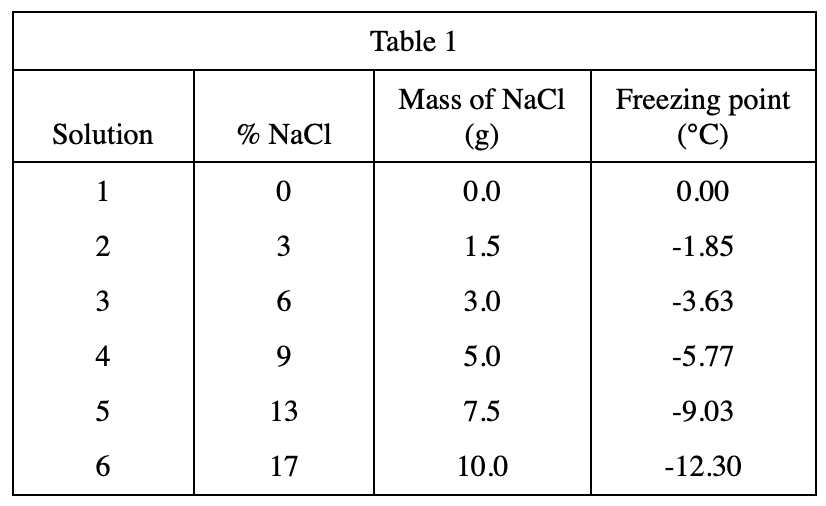
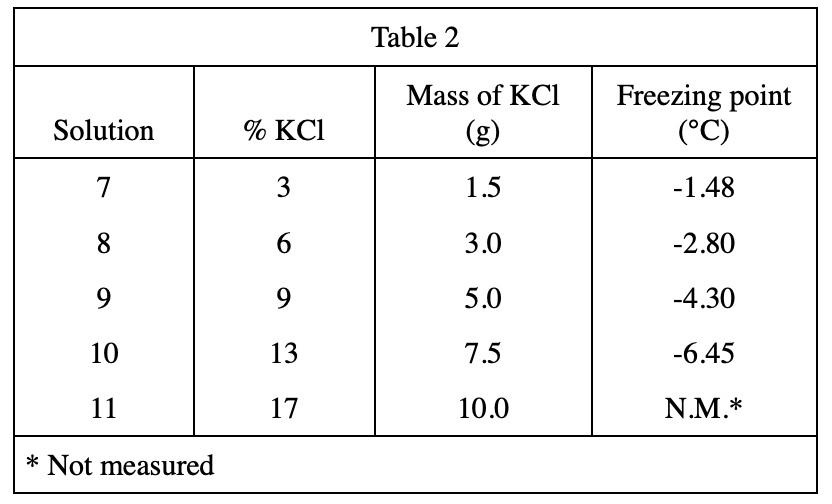
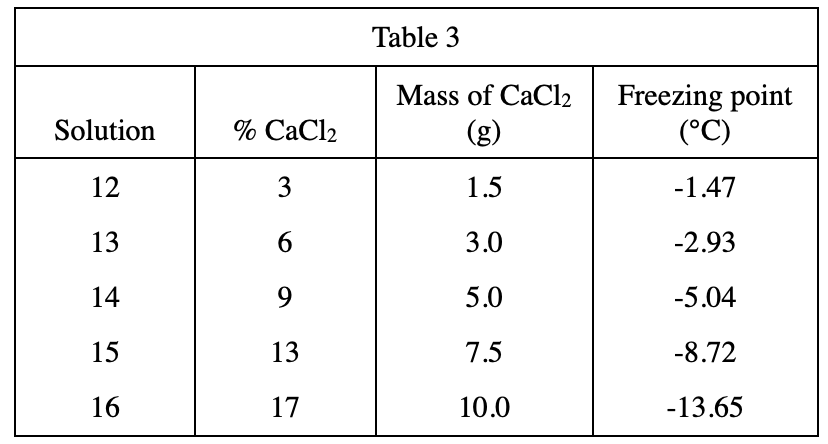
18. Which of the following assumptions did the chemist make about the dry ice used in Experiments 2 and 3 ?
Your Answer is
Correct Answer is H
Explanation
The purpose of the experimental design. Look at the second and third lines of the text part of Experiment 2. It is mentioned in the brackets that the temperature of dry ice is -70°C, which is used for bath, that is, to cool down the solutions. The experiment must assume that the freezing point of these solutions is higher than -70°C, otherwise the freezing point of some solutions cannot be measured, so choose H