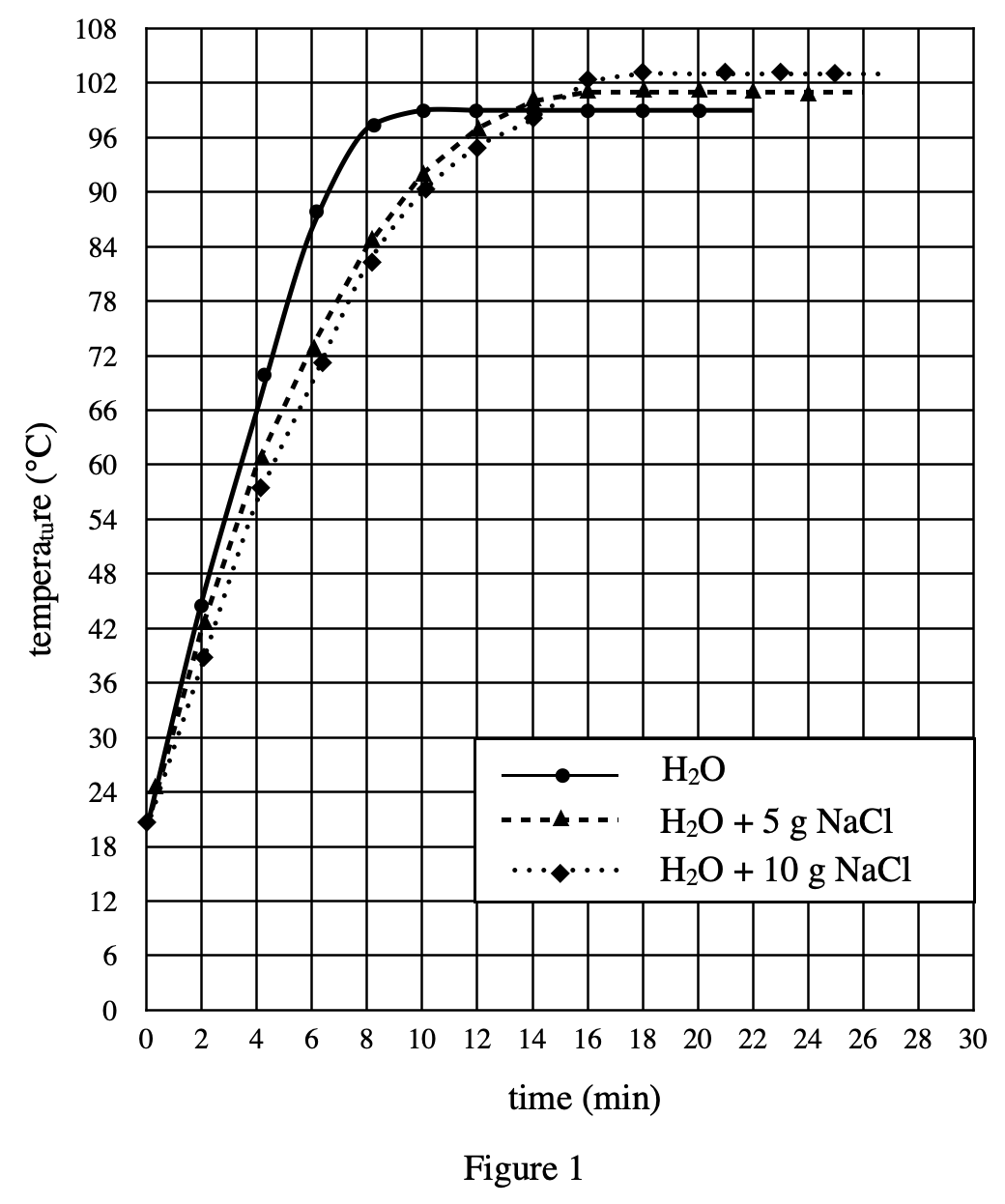
15. If, in Experiment 3, the chemist had measured the freezing point of Solution 11, the value would most likely be closest to:
Your Answer is
Correct Answer is B
Explanation
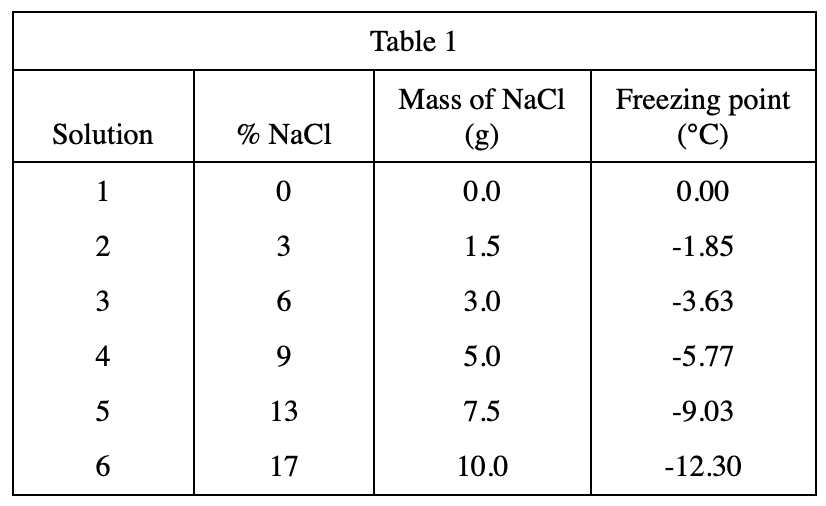
According to the data in table 2, it can be speculated that the higher the %KCl, the lower the freezing point.
The %KCl of Solution 11 is greater than the %KCl of Solution 10, so the freezing point of Solution 11 should also be lower than -6.45°C, so first exclude option A;
Then compare the table From Solution 9 and Solution 10 in 2, it can be seen that %KCl increases by 4%, and the freezing point decreases by about 2.15°C, so it is predicted that the freezing point of Solution 11 is about -6.45-2.15=-9, and option B is the closest