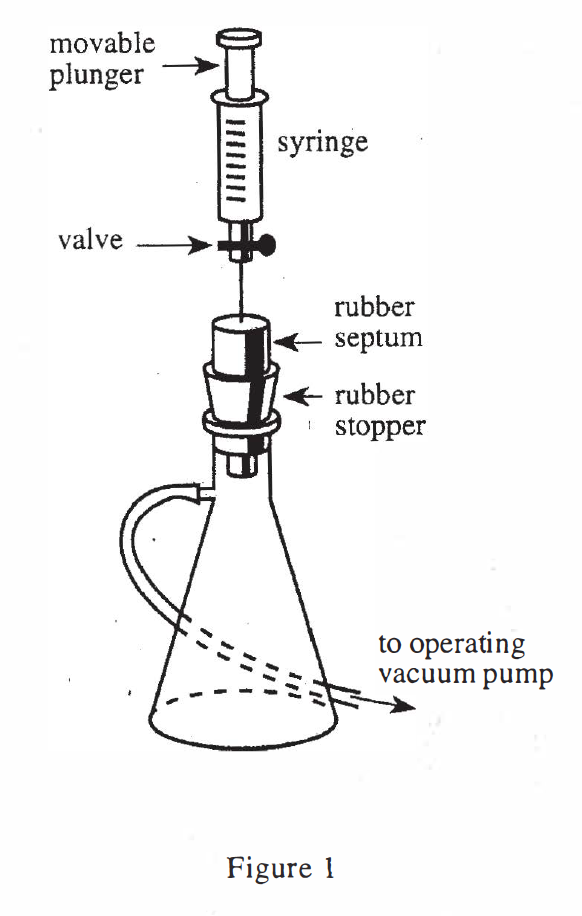
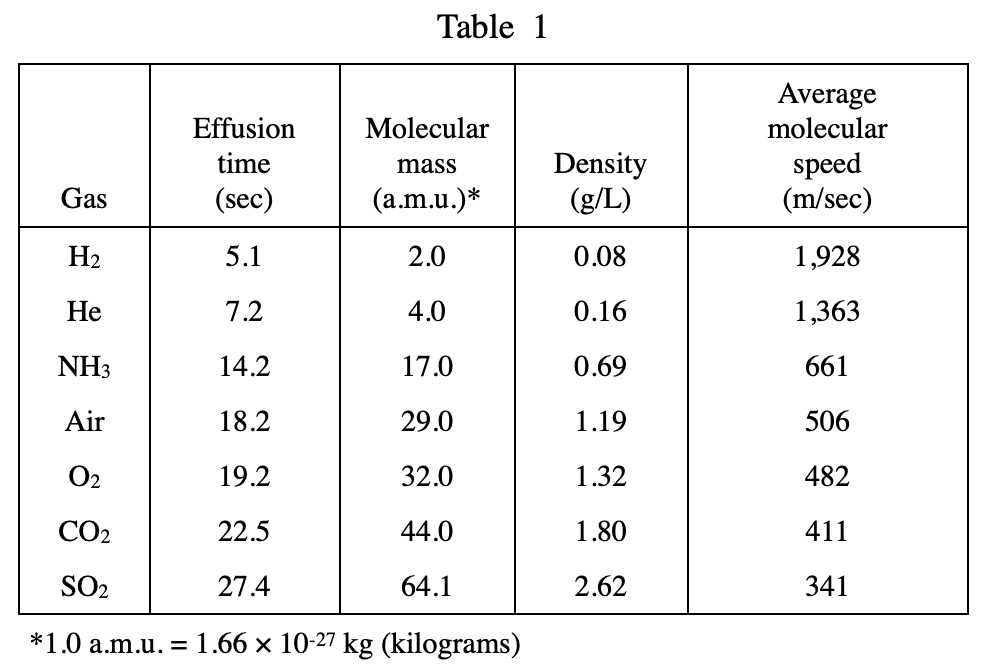
17. A 25 mL sample of CO2 gas is allowed to effuse in an apparatus like that in Figure 1. Given the information in Table 1, what percentage of the original number of CO2 gas molecules will remain in the syringe after 11.25 sec of effusion?
Your Answer is
Correct Answer is C
Explanation
Query table 1 and find that the time taken for 25 ml of CO2 to seep out is 22.5 seconds, half of 11.25 seconds, so after 11.25 seconds, half of the gas should still be in the syringe, so choose C