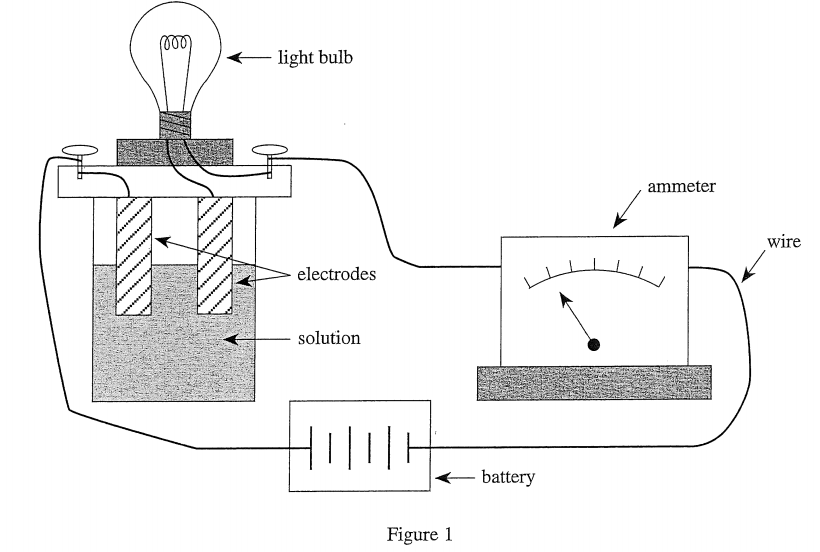
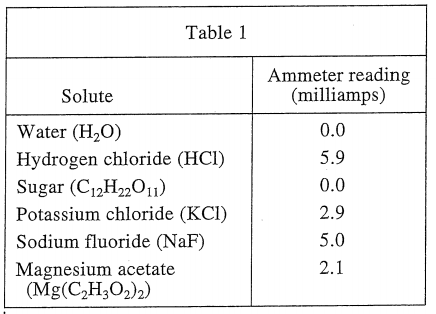
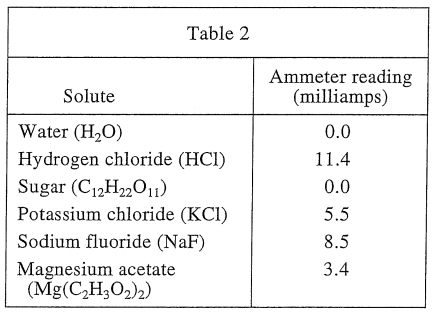
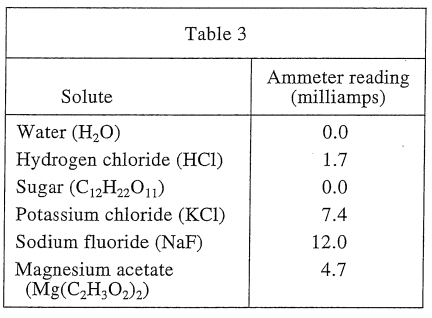
26. If a scientist dissolved both 10 g of C12H22O11 and 10 g of KCl in 100 mL of H2O at 50°C, the ammeter would read approximately:
Your Answer is
Correct Answer is H
Explanation
As can be seen from the three tables, sugar is not conductive when dissolved in water, so ignore it. Equivalent to 10 g of KCl dissolved in 100 mL of water at a temperature of 50°C, then you should see table 3, and the ammeter reading should be 7.4