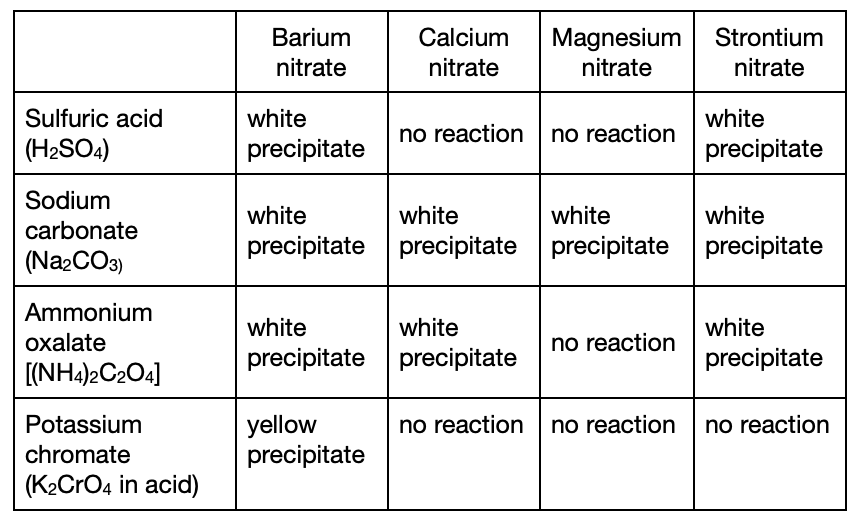
20. A student was given a solution that contained only one metallic ion, which was either Sr2+ or Ca2+. The student was told to run one test with only one reagent (a substance used to identify or produce other substances) to identify the ion. Which one of the following reagents should the student use to correctly identify the ion?
Your Answer is
Correct Answer is F
Explanation
You should choose a reagent that only reacts with one of Sr2+ or Ca2+ ions. According to the table, it should be sulfuric acid